Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the position of equilibrium for the following reaction. 
A) To the left
B) To the right
C) No reaction
Correct Answer

verified
Correct Answer
verified
Essay
In the following reaction,identify the acid and base as well as the conjugate acid and base. 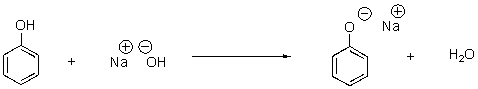
Correct Answer

verified
Acid,Base,...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Can this base exist in liquid ammonia? 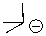
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Essay
Draw arrows to indicate the movement of electrons in the following reaction. 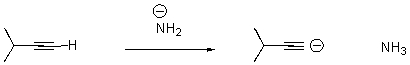
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the counterion of the t-butyl carbanion in the following compound? 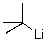
A) tert-butyl
B) C
C) Li+
D) H
Correct Answer

verified
Correct Answer
verified
Essay
What is the conjugate base of the following acid? 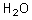
Correct Answer

verified
Correct Answer
verified
Essay
What is the difference between Ka and pKa?
Correct Answer

verified
pKa = -logKa
Correct Answer
verified
Multiple Choice
What is wrong with the following arrow? 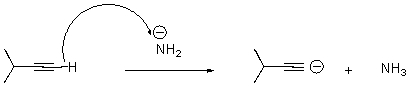
A) It should start on the alkyne carbon.
B) It should start on a hydrogen attached to the nitrogen.
C) It should start on the anion on nitrogen,end at the H on the alkyne,and a second arrow should start at the bond between the C and H on the alkyne and end on the terminal carbon of the alkyne.
D) There should be two arrows - one from nitrogen and one from the alkyne carbon.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Into which of the following categories does the following compound belong? 
A) Bronsted-Lowry Acid
B) Bronsted-Lowry Base
C) Lewis Acid
D) Lewis Base
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which side will the following acid-base reaction favor? 
A) The right
B) The left
C) Neither
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Could hydroxide deprotonate CH4?
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the position of equilibrium for the following reaction. 
A) To the left
B) To the right
C) No reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the position of equilibrium for the following reaction. 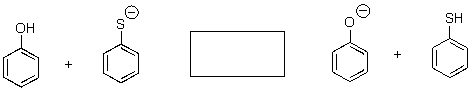
A) To the left
B) To the right
C) No reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Could water protonate the following compound? HOSO3-
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which side will the following acid-base reaction favor? 
A) The right
B) The left
C) Neither
Correct Answer

verified
A
Correct Answer
verified
Essay
In the following reaction,identify the Lewis acid and the Lewis base. 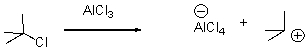
Correct Answer

verified
11eab5f2_25d2_b415_a628_8909408e1610_TB3186_00
Correct Answer
verified
Multiple Choice
Could the amide anion (- NH2) deprotonate CH4?
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Is the indicated compound acting an acid or a base in the following reaction? 
A) Acid
B) Base
C) Neither
Correct Answer

verified
Correct Answer
verified
Essay
Draw arrows to indicate the movement of electrons in the following reaction. 
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 72
Related Exams