A) When a reaction produces a mixture of enantiomers,it is often very difficult to separate them.A mixture of products is often not useful.
B) Often stereoisomers of a particular compound will have very different biological effects on an organism.Only one isomer is biologically helpful,and the other may be harmful.
C) Often only one stereoisomer is biologically active,and coupling reactions are often used for the production of biological materials.
D) All of the choices are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the structure of the organic product Y formed in the following reaction sequence. 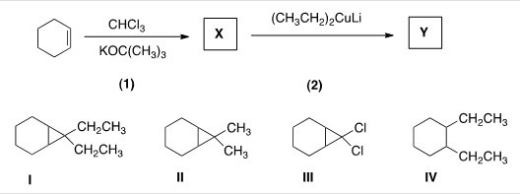
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below best explains what is meant by the statement,"An organocuprate reaction with a vinyl halide is stereospecific"?
A) The reaction of a specific stereoisomer with the R2CuLi reagent will yield that particular stereoisomer as the product.
B) The reaction of a vinyl halide with the R2CuLi reagent will only yield the cis product.
C) The reaction of a vinyl halide with the R2CuLi reagent will only yield the trans product.
D) The reaction of a vinyl halide with the R2CuLi reagent will only yield one enantiomer product-either R or S configuration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following descriptions does not apply to methylene?
A) Methylene is sp2 hybridized.
B) Methylene is a neutral,reactive intermediate.
C) Methylene is a radical intermediate.
D) The formula of methylene is :CH2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the Grubbs catalyst synthetically important?
A) Because it only requires dilute concentrations of the reactants
B) Because it produces only stereospecific products
C) Because it produces only stereoselective products
D) Because it provides a synthetic pathway for ring-closing metathesis reactions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the missing reactant in the following reaction? 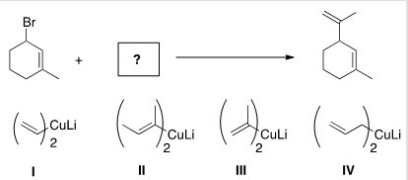
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do the Suzuki reaction,the Heck reaction,and the organocuprate reaction all have in common when they react with an alkyl halide?
A) All reactions form new carbon-carbon bonds.
B) They all use palladium as a catalyst in one step of the reaction.
C) They are all stereospecific reactions.
D) They all require harsh conditions.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 47 of 47
Related Exams