A) bromine with acetic acid.
B) bromine and aqueous hydroxide ion.
C) THF,LDA at -78 °C followed by reaction with bromine.
D) base and methyl bromide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ketones will give a positive iodoform test?
A) 3-Heptanone
B) 2-Pentanone
C) 3-Hexanone
D) Cyclohexanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vitamin C is a stable enediol.Which is the structure of a possible keto form in equilibrium with the enediol form? 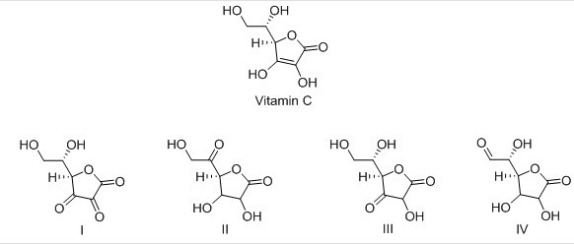
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the more stable form of acetophenone? 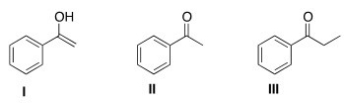
A) Only I
B) Only II
C) Only III
D) I and II are equally stable
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
If you want to form a thermodynamic enolate,you want to
A) keep the reaction as cold as possible.
B) use an aprotic solvent such as THF.
C) use a protic solvent such as ethanol.
D) use a carboxylic acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most stable form of 1,3-cyclohexanedione? 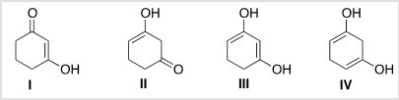
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of X,product of the following reaction? 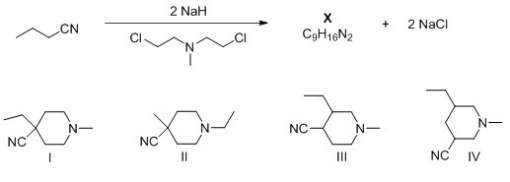
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the thermodynamic enolate of 2-methylcyclohexanone? 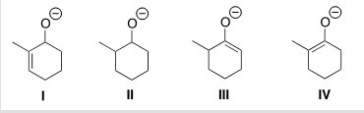
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the starting material in the following reaction? 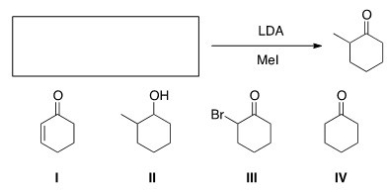
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 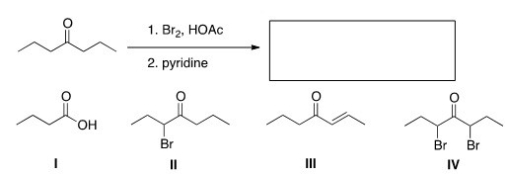
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction below is a direct enolate alkylation.It has been found that this reaction only works well with unhindered methyl and 1° alkyl halides.Pick the statement that best explains this observation. 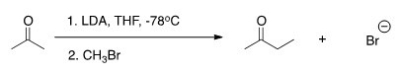
A) The nucleophilic enolate requires a reaction center that has a positive charge.
B) Hindered alkyl halides do not undergo SN1 reactions.
C) Hindered alkyl halides do not undergo SN2 reactions.
D) Methyl and 1° alkyl halides can form carbocations that can readily react with the nucleophilic enolate.
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 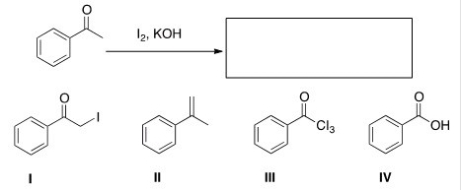
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a possible enol form of 2-butanone? 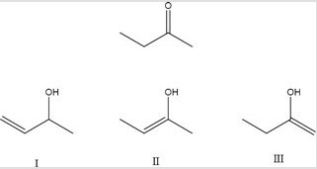
A) I
B) II
C) III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the starting material required to accomplish the following transformation? 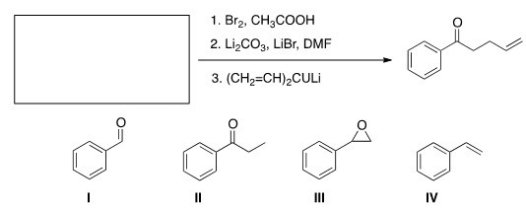
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is (are) the product(s) of the following reaction? 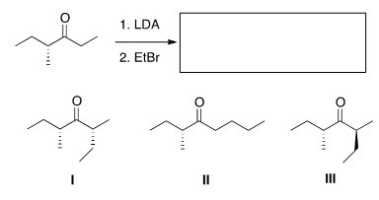
A) Only I
B) Only II
C) Only III
D) Only I and III
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Why is it difficult to stop the halogenation of ketones under basic conditions at the mono-halogenated stage?
A) The ketone undergoes a Bayer-Villigar oxidation.
B) The ketone is reduced.
C) The ketone undergoes an Aldol reaction.
D) The bromine helps to stabilize the second enolate,making the product more acidic than the starting material.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the appropriate sequence of reactions to accomplish the following synthesis. ![Select the appropriate sequence of reactions to accomplish the following synthesis. A) [1] Br<sub>2</sub>,CH<sub>3</sub>CO<sub>2</sub>H; [2] Li<sub>2</sub>CO<sub>3</sub>,LiBr,DMF; [3] CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>Br B) [1] Br<sub>2</sub>,CH<sub>3</sub>CO<sub>2</sub>H; [2] Mg,Et<sub>2</sub>O; [3] CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>Br C) [1] LDA; [2] BrCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>Br; [3] NaOEt D) [1] NaOEt; [2] BrCH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>Br; [3] LDA](https://d2lvgg3v3hfg70.cloudfront.net/TB7814/11eac680_9bde_e0f0_a448_1376f0cfa9c3_TB7814_00.jpg)
A) [1] Br2,CH3CO2H; [2] Li2CO3,LiBr,DMF; [3] CH3CH2CH2CH2Br
B) [1] Br2,CH3CO2H; [2] Mg,Et2O; [3] CH3CH2CH2CH2Br
C) [1] LDA; [2] BrCH2CH2CH2CH2Br; [3] NaOEt
D) [1] NaOEt; [2] BrCH2CH2CH2CH2Br; [3] LDA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Will acetone be completely deprotonated by potassium tert-butoxide?
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the intermediate for halogenation of ketones under acidic conditions?
A) An enolate
B) An enol
C) A tautomer
D) An epimer
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most acidic compound? 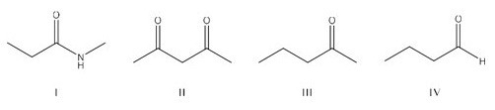
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 47
Related Exams