A) KI and O3
B) NaF and H2O
C) PCl5 and HF
D) Na2SO3 and BH3
E) RbBr and MgS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three nonequivalent Lewis structures for carbonyl sulfide,SCO,are given below.Use the concepts of formal charge and electronegativity to choose the structure that is the best representation. 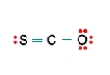 A
A 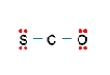 B
B 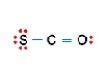 C
C
A) Structure A,because all the formal charges equal 0
B) Structure B,because all the formal charges equal 0
C) Structure C,because all the formal charges equal 0.
D) Structure A,because the negative formal charge resides on the most electronegative atom
E) Structure C,because the negative formal charge resides on the most electronegative atom
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many different molecules have the molecular formula C6H14?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a valid resonance structure for N3-?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) all are correct
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following molecules in order from smallest to largest H−N−H bond angles: NH4+,NH3,and NH2-.
A) NH4+ < NH3 < NH2-
B) NH4+ < NH2- < NH3
C) NH2- < NH3 < NH4+
D) NH2- < NH4+ < NH3
E) NH3 < NH2- < NH4+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One resonance structure for OCN- ion is drawn below.What is the formal charge on each atom? 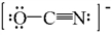
A) O atom = 0,C atom = 0,and N atom = 0
B) O atom = 0,C atom = 0,and N atom = -1
C) O atom = -1,C atom = 0,and N atom = 0
D) O atom = -1,C atom = -1,and N atom = +1
E) O atom = +1,C atom = 0,and N atom = -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure of which of the following molecules violates the octet rule?
A) SF4
B) NF3
C) OF2
D) HF
E) SiF4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms having equal or nearly equal electronegativities are expected to form
A) no bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) ionic bonds
E) covalent bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are the correct resonance structures for the formate ion,HCO2-? 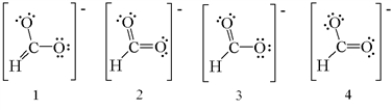
A) 1 and 2
B) 2 and 3
C) 3 and 4
D) 1,3,and 4
E) 2,3,and 4
Correct Answer

verified
Correct Answer
verified
Showing 81 - 89 of 89
Related Exams