A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of increasing dipole moment. I. BCl3 II. BIF2 III. BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the compound below that contains at least one polar covalent bond but is nonpolar.
A) GeH2Br2
B) SCl2
C) AsCl5
D) CF2Cl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set.
B) A bond order of 0 represents a stable chemical bond.
C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals.
D) Electrons placed in antibonding orbitals stabilize the ion/molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with trigonal bipyramidal electron geometry and linear molecular geometry.
A) 180°
B) <180°
C) >180°
D) <109.5°
E) <120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In molecular orbital theory the acronym LCAO stands for ________.
A) linear combination of atomic orbitals
B) lowest combined atomic orbitals
C) least consumed advanced orientation
D) linearly created available orbital
E) It has no meaning.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for COCl2. What is the hybridization on the C atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for S 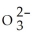 . What is the hybridization on the S atom?
. What is the hybridization on the S atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry (mg) of the bolded and underlined atom CH3CH2OH.
A) mg = tetrahedral
B) mg = trigonal pyramidal
C) mg = trigonal planar
D) mg = trigonal bipyramidal
E) mg = bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle in H2O.
A) 109.5°
B) 180°
C) 120.5°
D) 104.5°
E) 90.5°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule or ion with seven electrons in bonding orbitals and two electrons in an antibonding orbital has a bond order of ________.
A) 0.5
B) 1
C) 1.5
D) 2
E) 2.5
Correct Answer

verified
Correct Answer
verified
Essay
Is it possible for a molecule to be nonpolar even though it contains polar bonds? Explain your answer and give an example.
Correct Answer

verified
Yes. The polarity of a molecule depends ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Draw the Lewis structure for N2H2. What is the hybridization on the N atoms?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of Te Cl4?
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule PF3.
A) Trigonal pyramidal
B) Trigonal planar
C) Tetrahedral
D) T-shaped
E) Bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? XeO2 SiCl2Br2 C2Br2 SeCl6
A) 1
B) 4
C) 2
D) 3
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the approximate bond angle for a molecule with a tetrahedral shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of XeF4.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule below. Determine the molecular geometry at each of the two labelled carbons. 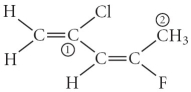
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2 = bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is least stable. 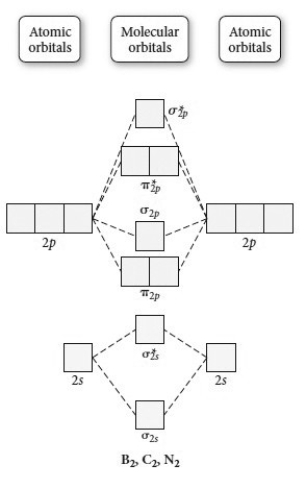
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B2⁺
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 180
Related Exams