A) C2H3O4
B) CHO2
C) C2H4O2
D) C2H2O4
E) CH2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percentage by mass of hydrogen in PtCl2(NH3) 2.
A) 1.008
B) 0.034
C) 2.016
D) 0.672
E) 1.558
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following equation is balanced, the coefficient of O2 is _ . 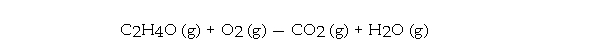
A) 4
B) 3
C) 2
D) 1
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of carbon dioxide are there in 52.06 g of carbon dioxide?
A) 0.8452
B) 1.183
C) 6.022 × 1023
D) 3.134 × 1025
E) 8.648 × 1023
Correct Answer

verified
Correct Answer
verified
Short Answer
The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide:
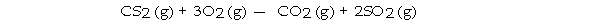 The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
Correct Answer

verified
Correct Answer
verified
Essay
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia:
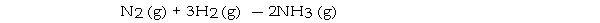 A 9.3- g sample of hydrogen requires
A 9.3- g sample of hydrogen requires
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is not true concerning automotive air bags?
A) The gas used for inflating them is oxygen
B) A gas is produced when the air bag activates.
C) They are loaded with sodium azide initially
D) The two products of the decomposition reaction are sodium and nitrogen
E) They are inflated as a result of a decomposition reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of propane (C3H8) produces CO2 and H2O:
 The reaction of 2.5 mol of O2 will produce mol of H2O.
The reaction of 2.5 mol of O2 will produce mol of H2O.
A) 4.0
B) 3.0
C) 2.5
D) 2.0
E) 1.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A nitrogen oxide is 63.65% by mass nitrogen. The molecular formula could be _ _.
A) N2O4
B) N2O
C) NO2
D) NO
E) either NO2 or N2O4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are _ sulfur atoms in 25 molecules of C4H4S2.
A) 3.0 × 1025
B) 50
C) 6.02 × 1023
D) 1.5 × 1025
E) 4.8 × 1025
Correct Answer

verified
Correct Answer
verified
True/False
Carbon dioxide called a greenhouse gas because bacterial degradation of fertilizers in a greenhouse environment produce large quantities of carbon dioxide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass. What is the empirical formula of the compound?
A) CH4
B) C2H4
C) CH2
D) C86H14
E) C4H8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percentage by mass of chlorine in PtCl2(NH3) 2.
A) 23.63
B) 12.53
C) 25.05
D) 11.82
E) 18.09
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many sulfur dioxide molecules are there in 1.80 mol of sulfur dioxide?
A) 1.80 × 1024
B) 6.02 × 1024
C) 6.02 × 1023
D) 1.08 × 1023
E) 1.08 × 1024
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum mass in grams of NH3 that can be produced by the reaction of 1.0 g of N2 with 3.0 g of H2 via the equation below? 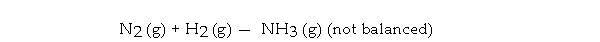
A) 0.61
B) 2.0
C) 17
D) 1.2
E) 4.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the reactions below, which one is a decomposition reaction?
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) 2Mg + O2 - 2MgO
C) Cd(NO3) 2 + Na2S - CdS + 2NaNO3
D) 2N2 + 3H2 - 2NH3
E) NH4Cl - NH3 + HCl
Correct Answer

verified
Correct Answer
verified
Short Answer
A compound was found to contain 90.6% lead (Pb) and 9.4% oxygen. The empirical formula for this compound is .
Correct Answer

verified
Correct Answer
verified
True/False
A great deal of the carbon dioxide produced by the combustion of fossil fuels is absorbed into the oceans.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass in grams of hydrogen is produced by the reaction of 4.73 g of magnesium with 1.83 g of water? 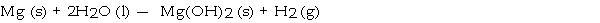
A) 0.0485
B) 0.219
C) 0.102
D) 0.0162
E) 0.204
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of nitrogen are in 10 g of NH4NO3?
A) 2
B) 3.0 × 1023
C) 1.8
D) 3.5
E) 1.5 × 1023
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 134
Related Exams