A) 546
B) 72.9
C) 273
D) 38
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a sample of gas (2.49 g) was 752 mL at 1.98 atm and 62 °C. The gas is .
A) Ne
B) NH3
C) NO2
D) SO2
E) SO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass of nitrogen dioxide contained in a 4.32 L vessel at 48 °C and 141600 Pa is _ _g)
A) 10.5
B) 5.35 × 104
C) 9.46 × 10- 2
D) 53.5
E) 70.5
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the partial pressure (in mm Hg) of neon in a 4.00 L vessel that contains 0.838 mol of methane, 0.184 mol of ethane, and 0.755 mol of neon at a total pressure of 928 mmHg?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, has the odor of rotting eggs.
A) NO2
B) NH3
C) CO
D) H2S
E) HCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of an unknown volatile liquid was injected into a Dumas flask (mflask = 27.0928 g, Vflask = 0.1040 L) and heated until no visible traces of the liquid could be found. The flask and its contents were then rapidly cooled and reweighed (mflask+vapor = 27.4593 g) . The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C, respectively. The unknown volatile liquid was .
A) C7H16
B) C6H6
C) C6H12
D) C7H14
E) C6H14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of fluorine gas required to react with 2.67 g of calcium bromide to form calcium fluoride and bromine at 41.0 °C and 4.31 atm is mL.
A) 104
B) 210
C) 10.4
D) 420
E) 79.9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pressure of a sample of CH4 gas (6.022 g) in a 30.0 L vessel at 402 K is _ atm.
A) 12.4
B) 0.414
C) 6.62
D) 2.42
E) 22.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask contains a mixture of He and Ne at a total pressure of 2.6 atm. There are 2.0 mol of He and 5) 0 mol of Ne in the flask. The partial pressure of He is atm.
A) 6.5
B) 1.86
C) 1.04
D) 9.1
E) 0.74
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a gas (5.0 mol) at 1.0 atm is expanded at constant temperature from 10 L to 15 L. The final pressure is atm.
A) 3.3
B) 1.5
C) 15
D) 7.5
E) 0.67
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Mond process produces pure nickel metal via the thermal decomposition of nickel tetracarbonyl:
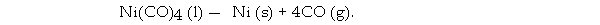 What volume (L) of CO is formed from the complete decomposition of 444 g of Ni(CO) 4 at 752 torr and 22.0 °C?
What volume (L) of CO is formed from the complete decomposition of 444 g of Ni(CO) 4 at 752 torr and 22.0 °C?
A) 20.2
B) 255
C) 0.356
D) 11.0
E) 63.7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of He gas (2.0 mmol) effused through a pinhole in 53 s. The same amount of an unknown gas, under the same conditions, effused through the pinhole in 248 s. The molecular mass of the unknown gas is _ g/mol.
A) 19
B) 5.5
C) 350
D) 88
E) 0.19
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas in a 325 mL container has a pressure of 695 torr at 19 °C. There are _ _ mol of gas in the flask.
A) 1.48 × 10- 2
B) 12.4
C) 1.24 × 10- 2
D) 80.6
E) 9.42
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of chlorine gas at 1.21 atm and 34.9 °C is g/L.
A) 1.70
B) 3.39
C) 0.0479
D) 0.295
E) 0.423
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas mixture of Ne and Ar has a total pressure of 4.00 atm and contains 16.0 mol of gas. If the partial pressure of Ne is 2.75 atm, how many moles of Ar are in the mixture?
A) 12.0
B) 11.0
C) 9.25
D) 5.00
E) 6.75
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture of two gases was allowed to effuse from a container. One of the gases escaped from the container 1.43 times as fast as the other one. The two gases could have been _.
A) CO and CO2
B) Cl2 and SF6
C) O2 and SF6
D) O2 and Cl2
E) CO and SF6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, is a valid statement of Charles' law.
A) ![]() constant
constant
B) ![]() constant
constant
C) PV = constant
D) V = constant × P
E) V = constant × n
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ammonium nitrite undergoes thermal decomposition to produce only gases:
 What volume (L) of gas is produced by the decomposition of 35.0 g of NH4NO2 (s) at 525 ° C and
1) 5 atm?
What volume (L) of gas is produced by the decomposition of 35.0 g of NH4NO2 (s) at 525 ° C and
1) 5 atm?
A) 47
B) 160
C) 24
D) 72
E) 15
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, _ is a correct statement of Boyle's law.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 50.75 g of a gas occupies 10.0 L at STP, 129.3 g of the gas will occupy L at STP.
A) 50.8
B) 25.5
C) 5.08
D) 12.9
E) 3.92
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 146
Related Exams