A) temperature increases.
B) volume increases.
C) pressure increases.
D) internal energy decreases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the second law of thermodynamics, which of the following applies to the heat received from a high temperature reservoir by a heat engine operating in a complete cycle?
A) must be completely converted to work
B) equals the entropy increase
C) converted completely into internal energy
D) cannot be completely converted to work
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On a P-V diagram, if a process involves a closed curve, the area inside the curve represents:
A) internal energy.
B) heat.
C) work.
D) zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an isothermal process where the pressure decreases from 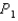 to
to 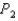 , does the internal energy of the system increase, decrease, or stay the same; and does the entropy of the system increase or decrease?
, does the internal energy of the system increase, decrease, or stay the same; and does the entropy of the system increase or decrease?
A) The internal energy decreases and the entropy increases.
B) The internal energy stays the same and the entropy increases.
C) The internal energy decreases and the entropy decreases.
D) The internal energy stays the same and the entropy decreases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an isothermal process for an ideal gas system (where the internal energy doesn't change) , which of the following choices best corresponds to the value of the work done by the system?
A) its heat intake
B) twice its heat intake
C) the negative of its heat intake
D) twice the negative of its heat intake
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an isochoric process where the pressure increases from 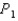 to
to 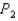 , does the internal energy of the system increase or decrease and does the temperature increase or decrease?
, does the internal energy of the system increase or decrease and does the temperature increase or decrease?
A) The internal energy increases and the temperature decreases.
B) The internal energy increases and the temperature increases.
C) The internal energy decreases and the temperature decreases.
D) The internal energy decreases and the temperature increases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 4-mol ideal gas system undergoes an adiabatic process where it expands and does 28 J of work on its environment. How much heat is received by the system?
A) "-20 J"
B) "zero"
C) "+28 J"
D) "+7 J"
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following heat engines, which has the highest efficiency?
A) Hero's engine
B) a Carnot engine
C) a car's gasoline engine
D) a truck's diesel engine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The adiabatic index, 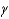 , of a gas is given by which of the following?
, of a gas is given by which of the following?
A) CP /CV
B) CV /CP
C) CP - CV
D) CP + CV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which system is heat usually transferred from the cooler part to the warmer part?
A) a stove as it heats up water
B) a refrigerator that is running
C) an electric fan that is running
D) none of the above, because it is impossible to transfer heat in this manner
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.0-kg chunk of ice at 0 C melts, absorbing 80,000 cal of heat in the process. Which of the following best describes what happens to this system?
A) increased entropy
B) lost entropy
C) entropy maintained constant
D) work converted to energy
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an isobaric process 3.6 * 104 J of work is done on a quantity of gas while its volume changes from 2.3 m3 to 1.1 m3. What is the pressure during this process?
A) 1.2 * 104 Pa
B) 2.4 * 104 Pa
C) 3.0 *104 Pa
D) 8.3 *104 Pa
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose a power plant uses a Carnot engine to generate electricity, using the atmosphere at 300 K as the low-temperature reservoir. Suppose the power plant produces an amount of electricity with the hot reservoir at 500 K during Day One and then produces the same amount of electricity with the hot reservoir at 600 K during Day Two. The thermal pollution was:
A) greatest on Day One.
B) greatest on Day Two.
C) the same on both days.
D) zero on both days.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The efficiency of a Carnot engine operating between 100 C and 0 C is most nearly:
A) 7%.
B) 15%.
C) 27%.
D) 51%.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On a PV diagram, 2 curves are plotted, both starting at the same point (P1, V1) and both ending at the same increased volume (V2) . One curve is for an isothermal process; the other is for an adiabatic process. Except for the common starting point, which curve is the upper one?
A) The isothermal process curve will always be the upper one.
B) The adiabatic process curve will always be the upper one.
C) Since they start at the same point and end at the same volume, they will coincide.
D) The isothermal one will start out higher, but the adiabatic one will eventually cross it.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 600-MW electric power plant has an efficiency of 30%. It loses its waste heat in large cooling towers. Approximately how much waste heat (in MJ) is discharged to the atmosphere per second?
A) 1400 MJ
B) 1900 MJ
C) 800 MJ
D) 560 MJ
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The maximum theoretical thermodynamic efficiency of a heat engine operating between hot and cold reservoirs is a function of which of the following?
A) hot reservoir temperature only
B) cold reservoir temperature only
C) both hot and cold reservoir temperatures
D) None of the above choices are valid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the first law of thermodynamics, for any process that may occur within an isolated system, which of the following choices applies?
A) entropy remains constant
B) entropy increases
C) entropy decreases
D) None of the above choices apply.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A 2.00-kg block of ice is at STP (0 C, 1 atm) while it melts completely to water. What is its change in entropy? (For ice, Lf = 3.34 *105 J/kg)
A) zero
B) 584 J/K
C) 1220 J/K
D) 2450 J/K
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A heat engine operating between a pair of hot and cold reservoirs with respective temperatures of 500 K and 300 K will have what maximum efficiency?
A) 60%
B) 50%
C) 40%
D) 30%
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 68
Related Exams