Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure shown below is not a valid Lewis structure. What statement best describes the error in the structure? 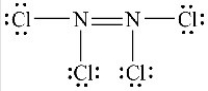
A) The nitrogen atoms violate the octet rule.
B) The chlorine atoms violate the octet rule.
C) The structure contains an incorrect number of valence electrons.
D) Chlorine atoms and nitrogen atoms do not typically form bonds with each other.
Correct Answer

verified
Correct Answer
verified
Short Answer
Carbon usually forms four bonds in stable molecules. However unstable carbon compounds with less than four bonds are known. The methyl carbanion shown below is an example. The molecular shape around the carbon atom in this structure is ________. 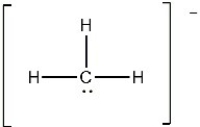
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct Lewis structure for OBr-?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure of formaldehyde is shown below. Which statement concerning this structure is INCORRECT? 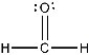
A) Two electrons are being shared between the carbon atom and the oxygen atom.
B) The oxygen atom has four valence electrons that are not being shared with another atom.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have filled valence shells.
Correct Answer

verified
Correct Answer
verified
True/False
Some covalent compounds are solids,some are liquids,and some are gases at room temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon tetrachloride has ________ valence electrons.
A) 4 (four)
B) 8 (eight)
C) 24 (twenty-four)
D) 32 (thirty-two)
E) 40 (forty)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which choice would be named carbon tetrachloride?
A) CCl
B) C4Cl
C) CCl4
D) CCl3
E) C3Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is classified as a group in the valence shell electron pair repulsion (VSEPR) theory?
A) An atom
B) A lone pair of electrons
C) A valence electron
D) Either an atom or a valence electron
E) Either an atom or a lone pair of electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule's Lewis structure contains an atom that violates the octet rule?
A) H2O
B) BeH2
C) PCl3
D) H2Se
Correct Answer

verified
Correct Answer
verified
True/False
Nonpolar molecules may contain polar bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning chemical bonds is FALSE?
A) A covalent bond involves the sharing of electrons between two nonmetal atoms.
B) An ionic bond is the attraction between oppositely charged ions.
C) A nonpolar bond is a covalent bond in which electrons are not shared equally between the atoms.
D) Electronegativity is a measure of the attraction an atom has for the electrons it shares in a bond.
Correct Answer

verified
Correct Answer
verified
True/False
When writing Lewis structures,the symbol below is placed between resonance structures. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements will generally form only one covalent bond?
A) Sulfur
B) Carbon
C) Hydrogen
D) Argon
E) Nitrogen
Correct Answer

verified
Correct Answer
verified
True/False
A N-O bond is more polar than a P-O bond.
Correct Answer

verified
Correct Answer
verified
True/False
Atoms with three valence electrons generally form five bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many covalent bonds are generally formed by an oxygen atom?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
True/False
C-H bonds are considered to be nonpolar,because the electronegativity difference between carbon and hydrogen is small.
Correct Answer

verified
Correct Answer
verified
True/False
In the valence shell electron pair repulsion (VSEPR)theory,a group is defined as an atom or a lone pair of electrons.
Correct Answer

verified
Correct Answer
verified
True/False
Double bonds and triple bonds are never polar bonds.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 101
Related Exams