A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound has five singlets in its proton-decoupled 13C NMR spectrum and two quartets, one triplet, one doublet, and one singlet in its proton-coupled 13C NMR spectrum?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the wavelength of the radiation increases, the _______ decreases.
A) energy
B) frequency
C) wave number
D) all of these
E) all increase
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many peaks would you expect in the proton decoupled 13C NMR spectrum of 2-pentanol?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following forms of electromagnetic radiation has the highest energy?
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond would show a strong, sharp absorption in the IR between 1200 and 1000 cm-1?
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound 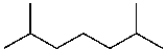 will show what number of peaks in its 1H decoupled 13C NMR spectrum?
will show what number of peaks in its 1H decoupled 13C NMR spectrum?
A) 3
B) 4
C) 5
D) 6
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A monochloroalkane shows two parent ion peaks m/z at 92 and 94.What is the molecular formula?
A) C4H9Cl
B) C3H7Cl
C) C3H21Cl
D) C4H7Cl
E) C2H6Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown hydrocarbon shows a parent peak on its mass spectra at m/z 86.There is no evidence of absorptions from visible or uv spectra.The IR shows no major absorptions outside the C-H stretch and bending vibrations.The 1H NMR reveals a singlet at 0.9 (9H) , a triplet at 0.98 (3H) , and a quartet at 1.6 (2H) .
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The IR spectrum stretching frequency that has the highest energy occurs for which of these bonds?
A) O-H
B) C-O
C) C-Cl
D) C-H
E) C-C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond would show a sharp absorption in the IR between 3000 and 2800 cm-1?
A) O-H
B) N-H
C) C=O
D) C-H
E) C-O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown oxygen containing organic molecule shows a parent peak in its mass spectrum at m/z 58.There is no measurable uv or visible spectrum.The IR shows strong, sharp absorptions at 2930 cm-1 and 1725 cm-1.The 1H NMR shows a singlet at 2.1 (6H) .Which of the following structures best fits the spectral data?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following forms of electromagnetic radiation has the longest wavelength?
A) radiowaves
B) infrared
C) visible
D) uv
E) x-ray
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds will have two peaks in its 1H NMR spectrum and a broad band in the 3200 to 3500 cm-1 region of its IR spectrum?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many peak(s) would you expect to see in the 1H NMR spectrum of 1-bromobutane?
A) 1
B) 2
C) 3
D) 4
E) more than 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules shows only one singlet in its 1H NMR spectrum? 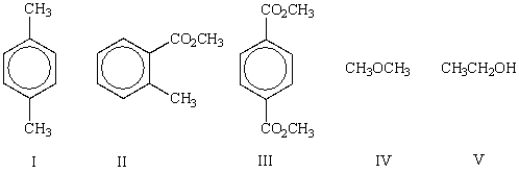
A) I
B) II
C) I and III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An IR spectrum of an unknown organic molecule having the formula C3H6O2 reveals strong absorptions at 2500-3400 cm-1, 1715 cm-1, and 1230 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) carboxylic acid
C) aldehyde
D) ester
E) ether
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An IR spectrum of an unknown organic molecule having the formula C3H6O reveals a strong absorption at 1230 cm-1 and has no absorption over 3000 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An IR spectrum of an unknown organic molecule having the formula C2H4O reveals a strong absorption at 1715-1725 cm-1.To what class of compounds does the unknown belong?
A) alcohol
B) amine
C) aldehyde
D) alkyne
E) ether
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectrum of 1-pentanol shows an intense daughter ion peak at m/z = 31.This peak could be due to the formation of:
A) C5H12O•
B) C3H7O+
C) CH3O+
D) CH4O•
E) C4H10O+
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 48
Related Exams