A) 1 × 10-2
B) 1 × 102
C) 1 × 10-11
D) 1 × 1011
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which K value indicates a reaction that MOST favors the products?
A) 1 × 10-2
B) 1 × 102
C) 1 × 10-11
D) 1 × 1011
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the number 4 corresponds to the: 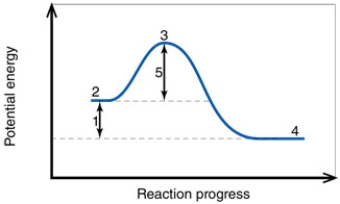
A) reactants.
B) products.
C) activation energy.
D) transition state.
E) net energy change.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which choice is NOT included in the equilibrium expression?
A) solids
B) gases
C) aqueous solutions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the net energy change is about: 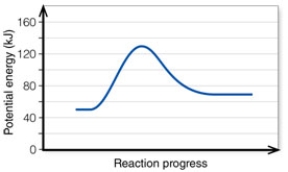
A) 60 kJ.
B) 130 kJ.
C) 80 kJ.
D) 20 kJ.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the activation energy of the FORWARD reaction is about: 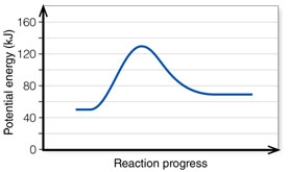
A) 60 kJ.
B) 130 kJ.
C) 80 kJ.
D) 20 kJ.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium expression of the following reaction is represented by: H2 (g) + I2 (g) 2 HI (g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
In a saturated solution of aluminum phosphate, AlPO4, both [Al3+] and [PO43-] = 7.9 × 10-10 M. Calculate the value of the solubility product for this compound.
A) 6.2 × 10-19
B) 2.8 × 10-5
C) 1.6 × 10-9
D) 4.0 × 10-10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the FORWARD reaction is: 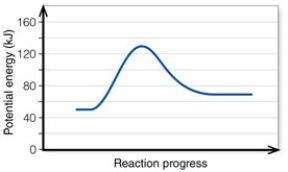
A) endothermic.
B) exothermic.
C) exergetic.
D) ergonomic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the number 1 corresponds to the: 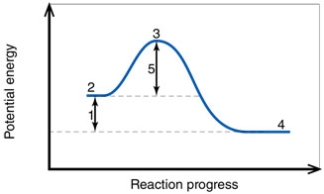
A) reactants.
B) products.
C) activation energy.
D) transition state.
E) net energy change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the solubility product expression for the following solubility equilibrium: CuOH (s) Cu+ (aq) + OH- (aq)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium expression of the following reaction is represented by: 2 CO (g) + O2 (g) 2 CO2 (g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the number 5 corresponds to the: 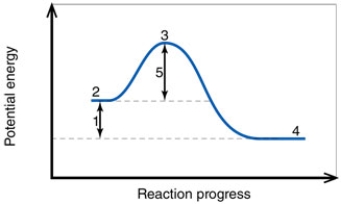
A) reactants.
B) products.
C) activation energy.
D) transition state.
E) net energy change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the energy of the transition state is about: 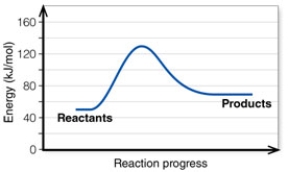
A) 50 kJ/mol.
B) 130 kJ/mol.
C) 80 kJ/mol.
D) 20 kJ/mol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following equation and equilibrium concentrations, determine the value of the equilibrium constant. PCl5 (g) PCl3 (g) + Cl2 (g) [PCl5] = 5.30 M; [PCl3] = 0.406 M; [Cl2] = 0.406 M
A) 32.2
B) 0.0311
C) 6.52
D) 0.153
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the equilibrium reaction between the gas phases of hydrogen and iodine: H2 (g) + I2 (g) 2 HI (g) If the partial pressures of hydrogen and of iodine are 0.42 atm each, what is the partial pressure of HI if K = 7.1 × 102?
A) 11 atm
B) 63 atm
C) 125 atm
D) 2.5 × 10-4 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the forward reaction will probably not proceed without an input of energy because the: 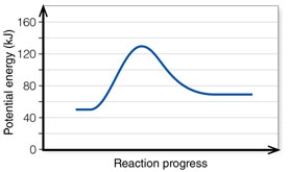
A) activation energy is too high.
B) net energy change is too low.
C) products are too far apart from the reactants.
D) reactant energy is too high to start with.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram, the reaction described is _____ and the equilibrium favors the _____. 
A) endothermic; reactants
B) endothermic; products
C) exothermic; reactants
D) exothermic; products
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The symbol used to represent the equilibrium solubility of a slightly soluble compound in water is:
A) Ka.
B) Kb.
C) Ks.
D) Ksp.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Considering the solubility product values for the following carbonates, which is the LEAST soluble?
A) CuCO3Ksp = 1.4 × 10-10
B) FeCO3 Ksp = 3.2 × 10-11
C) PbCO3Ksp = 7.4 × 10-14
D) MgCO3Ksp = 3.5 × 10-8
Correct Answer

verified
C
Correct Answer
verified
Showing 1 - 20 of 40
Related Exams