A) 1642 J
B) 2358 J
C) 1483 J
D) 2517 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas follows the pV trajectory shown in Figure 16.2. How much work is done per cycle by the gas if The gas in a heat engine follows the cycle shown in the pV diagram. How much work does this engine do each cycle if p0 = 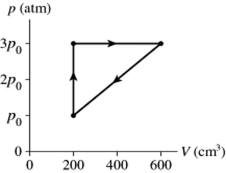
A) 220 J
B) 440 J
C) 870 J
D) 1100 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An athlete doing push-ups performs 650 kJ of work and loses 425 kJ of heat. What is the change in the internal (thermal) energy of the athlete?
A) -225 kJ
B) -1075 kJ
C) 1075 kJ
D) 225 kJ
E) 276 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A refrigerator has a coefficient of performance equal to 4.2. How much work must be done on the operating gas in the refrigerator in order to remove 250 J of heat from the interior compartment?
A) 60 J
B) 120 J
C) 250 J
D) 480 J
E) 1050 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An important feature of the Carnot cycle is that
A) its efficiency can be 100%.
B) its efficiency depends only on the absolute temperature of the hot reservoir used.
C) its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used, and on nothing else.
D) it is an example of an irreversible process that can be analyzed exactly without approximations.
E) no engine can be more efficient than a Carnot engine operating between the same two temperatures.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An expandable container holds 2.30 mole of helium, He, gas with an initial pressure of 770 kPa and an initial volume of 2.10 L. The gas expands isothermally to a final pressure of 350 kPa. How much heat is gained by the gas during this process? (R = 8.31 J/mol ∙ K)
A) 1280 J
B) 792 J
C) 685 J
D) 1370 J
E) 1700 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A temperature change of 20 C° corresponds to a Fahrenheit temperature change of
A) 68 F°.
B) 11 F°.
C) 36 F°.
D) 18 F°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A heat pump with a performance coefficient (COP) of 4.9 absorbs heat from the atmosphere at a rate of At what rate is work being done to run this heat pump?
A) 6 kW
B) 142 kW
C) 113 kW
D) 35 kW
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Express -40°C in °F.
A) -72°F
B) -54°F
C) -40°F
D) 4.4°F
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During an isothermal process, 5.0 J of heat is removed from an ideal gas. What is the work done by the gas in the process?
A) 0 J
B) 5.0 J
C) -5.0 J
D) -10 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A nuclear power plant has an actual efficiency of 33%. If of energy are released from fission, how much electric power does the power plant produce?
A) 0.063 MW
B) 6.3 MW
C) 25 MW
D) 0.25 MW
Correct Answer

verified
Correct Answer
verified
Short Answer
An ideal Carnot engine is operated between a hot and a cold reservoir. The temperature difference between the two reservoirs is 284°C. If the efficiency of this ideal engine is 24.0%, find the temperature of the cold reservoir in degrees Celsius.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents 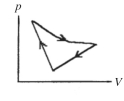
A) the heat that flows out of the gas.
B) the work done on the gas.
C) the heat added to the gas.
D) the work done by the gas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In the figure, Pa = Pc = 240 kPa, Vb = Vc = 40 L, Va = 15 L, and Pb = 400 kPa. How much heat is gained by the gas in this a→b→c→a process? 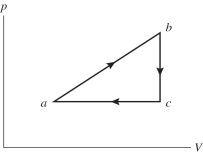
A) 1000 J
B) 1500 J
C) 2000 J
D) 2500 J
E) 3000 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During each cycle, the compressor in a certain ideal Carnot refrigerator performs 480 J of work to remove 150 J of heat from the interior of the refrigerator. How much heat do the coils behind the refrigerator discharge into the kitchen each cycle?
A) 110 J
B) 150 J
C) 330 J
D) 480 J
E) 630 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the efficiency of an ideal Carnot engine operating between a reservoir in which ice and water coexist, and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atm for both reservoirs.
A) 27%
B) 0.27%
C) 100%
D) 1.0%
E) 15%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A heat engine receives 7000 J of heat and loses 3000 J in each cycle. What is the efficiency of this engine?
A) 57%
B) 30%
C) 70%
D) 43%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sealed rigid tank contains 29 moles of an ideal gas, at an initial temperature of The pressure of the gas is increased until the final pressure equals 1.90 times the initial pressure. The heat capacity at constant pressure of the gas is How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ? K)
A) 110 kJ
B) 170 kJ
C) 230 kJ
D) -52 kJ
E) 7.0 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ideal Carnot engine operates between a warm reservoir at 233 K and a colder reservoir. During each cycle, this engine extracts of heat from the warm reservoir and does of work. What is the temperature of the colder reservoir?
A) 171 K
B) 62 K
C) 47 K
D) 67 K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the efficiency of a Carnot engine were to be 100%, the heat sink would have to be
A) at absolute zero.
B) at 0°C.
C) at 100°C.
D) infinitely hot.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 106
Related Exams