A) one
B) two
C) three
D) four
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure (first three rows of the periodic table) to answer the questions below.
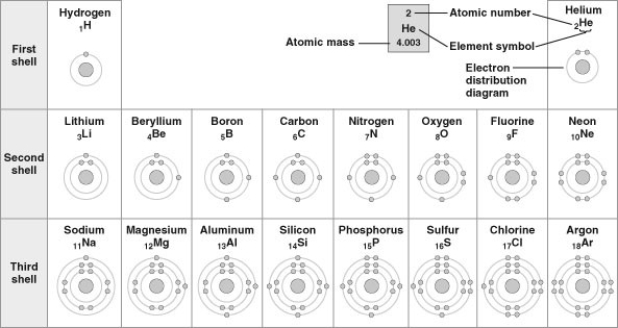 -What element does not prefer to react with other elements?
-What element does not prefer to react with other elements?
A) hydrogen
B) helium
C) beryllium
D) both hydrogen and beryllium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
B) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
D) Covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is broken when water evaporates?
A) nonpolar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) polar covalent bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best description of an atom's physical structure?
A) An atom is a solid mass of material.
B) The particles that form an atom are equidistant from each other.
C) Atoms are little bubbles of space with mass concentrated at the center of the bubble.
D) Atoms are little bubbles of space with mass concentrated on the outside surface of the bubble.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of chlorine is 17. The atomic number of magnesium is 12. What is the formula for magnesium chloride?
A) MgCl
B) MgCl₂
C) Mg₂Cl
D) MgCl₃
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen (N) normally forms three covalent bonds with a valence of five. However, ammonium has four covalent bonds, each to a different hydrogen (H) atom (H has a valence of one) . What do you predict to be the charge on ammonium?
A) +1
B) -1
C) +2
D) -2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound.
A) HS
B) HS₂
C) H₂S
D) H₄S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
 -If an atom has a charge of +1, which of the following must be true?
-If an atom has a charge of +1, which of the following must be true?
A) It has two more protons than neutrons.
B) It has the same number of protons as electrons.
C) It has one more electron than it does protons.
D) It has one more proton than it does electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
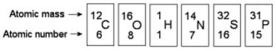 -How many electrons are present in a Phosphorus 3+ atom?
-How many electrons are present in a Phosphorus 3+ atom?
A) 16
B) 12
C) 19
D) 34
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
 -What causes the shape of the molecule shown?
-What causes the shape of the molecule shown?
A) the shape of the two p orbitals in the carbon atom
B) the shape of the one s orbital in the carbon atom
C) the shape of the sp³ hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) hydrogen bonding configurations between the carbon and hydrogen atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
 -An atom has four electrons in its valence shell. What types of covalent bonds is it capable of forming?
-An atom has four electrons in its valence shell. What types of covalent bonds is it capable of forming?
A) single, double, or triple
B) single and double only
C) single bonds only
D) double bonds only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the term trace element, the adjective trace means that
A) the element is required in very small amounts.
B) the element can be used as a label to trace atoms through an organism's metabolism.
C) the element is very rare on Earth.
D) the element enhances health but is not essential for the organism's long-term survival.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A neutral atom has two, eight, eight electrons in its first, second, and third energy levels. This information ________.
A) does not tell us about the atomic number of the element
B) does not tell us about the chemical properties of the element
C) does not tell us about the atomic mass of the element
D) does not tell us about the size of the element
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes any chemical reaction that has reached equilibrium?
A) The concentrations of products and reactants are equal.
B) The reaction is now irreversible.
C) Both forward and reverse reactions have halted.
D) The rates of the forward and reverse reactions are equal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure (first three rows of the periodic table) to answer the questions below.
 -Which pair of elements would likely have similar valency and thus similar chemical behavior?
-Which pair of elements would likely have similar valency and thus similar chemical behavior?
A) nitrogen and phosphorus
B) carbon and nitrogen
C) sodium and chlorine
D) hydrogen and helium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
 -Based on electron configuration, which of the elements in the figure would exhibit a chemical behavior most like that of oxygen?
-Based on electron configuration, which of the elements in the figure would exhibit a chemical behavior most like that of oxygen?
A) carbon
B) nitrogen
C) sulfur
D) phosphorus
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A covalent chemical bond is one in which ________.
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom
Correct Answer

verified
Correct Answer
verified
Multiple Choice
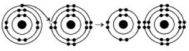
Refer to the following figure to answer the questions below.
 -What results from the chemical reaction in the illustration? The reactants have no charge.
-What results from the chemical reaction in the illustration? The reactants have no charge.
A) a cation with a net charge of +1 and an anion with a net charge of +1
B) a cation with a net charge of -1 and an anion with a net charge of -1
C) a cation with a net charge of -1 and an anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of -1
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 61
Related Exams