Correct Answer

verified
CH3CH2CH2CH3 has greater van der W...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the structure for isopentyl alcohol.
Correct Answer

verified
Correct Answer
verified
Essay
Draw a Newman projection for a gauche conformer of butane viewed along the C2-C3 bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds cannot form hydrogen bonds between its molecules?
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why primary and secondary amines form hydrogen bonds but tertiary amines do not.
Correct Answer

verified
The nitrogen in a tertiary ami...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
If an acyclic alkane contains n carbons, how many hydrogens does it contain?
A) n
B) n+2
C) n-2
D) 2n
E) 2n + 2
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Newman projection for the most stable conformer of 2-methylpropane.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformer of trans-1-ethyl-2-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular formula of the following compound?
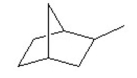
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which conformers of butane, viewed along the Cl-C2 bond, has the lowest energy?
A) the gauche conformer
B) the eclipsed and gauche conformers
C) the anti conformer
D) the eclipsed conformer
E) the anti and gauche conformers
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following compounds has the lowest boiling point?
A)
B) ![]()
C) ![]()
D) ![]()
E) CH3CH2CH2CH2CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes the most stable conformer of methylcyclohexane?
A) Both groups are equatorial.
B) Both groups are axial.
C) The tert-butyl group is equatorial and the methyl group is axial.
D) The tert-butyl group is axial and the methyl group is equatorial.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest boiling point?
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is sec-butyl alcohol?
A)
B)
C)
D)
E)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformer of cis-1-tert-butyl-4-methylcyclohexane.
Correct Answer

verified
11eb4044_1de5_b848_ad6e_15b83e0e7780_TB1829_00
Correct Answer
verified
Multiple Choice
Which conformer has the greatest energy in the potential energy diagram for chair-chair interconversion of cyclohexane?
A) twist-boat
B) planar
C) boat
D) half-chair
E) chair
Correct Answer

verified
D
Correct Answer
verified
Essay
Would you expect sodium chloride (NaCl) to be soluble in hexane? Explain your answer.
Correct Answer

verified
One would not expect NaCl to b...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Name the cycloalkane shown below. 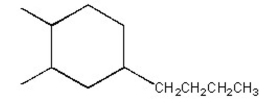
Correct Answer

verified
4-butyl-1,...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which compound is more soluble in water? Explain your choice. CH3OCH3 or CH3CH2OH
Correct Answer

verified
CH3CH2OH is more soluble in wate...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How many tertiary carbons are in the following compound? 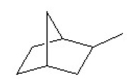
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 102
Related Exams