A) I < II < III
B) II < I < III
C) I < III < II
D) II < III < I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained in the following reaction?
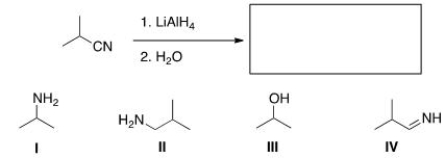
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound with molecular formula C6H15N exhibits a singlet at d 0.9 (1H) , a triplet at d 1.10 (3H) , a singlet at d1.15 (9H) , and a quartet at d 2.6 (2H) in its 1HNMR spectrum.Its IR spectrum shows one medium absorption band near 3400 cm-1.What is the structure of this compound?
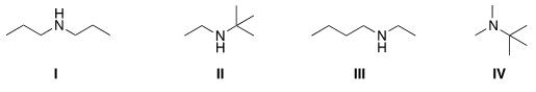
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the nitrogen atoms in chloroquine, shown below, in order of decreasing basicity, putting the most basic nitrogen atom first. 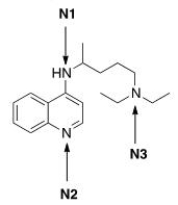
A) N1 > N2 > N3
B) N2 > N1 > N3
C) N3 > N2 > N1
D) N3 > N1 > N2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the preparation of primary amines, how can direct nucleophilic substitution between NH3 and alkyl halide be made more practical than reacting NH3 and the alkyl halide in a 1:1 ratio?
A) Use a large excess of NH3.
B) Use a large excess of alkyl halide.
C) Use a limited amount of NH3.
D) Make the alkyl halide sterically hindered.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound?
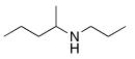
A) 1-methyl-N-propyl-1-propanamine
B) 4-methyl-4-heptanamine
C) 2-propyl-3-hexanamine
D) N-propyl-2-pentanamine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of the following compound?
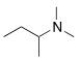
A) dimethylisobutylamine
B) butyldimethylamine
C) N,N-dimethylbutanamine
D) sec-butyldimethylamine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound?
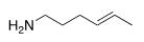
A) (Z) -4-hexen-1-amine
B) (E) -4-hexen-1-amine
C) (E) -2-hexen-6-amine
D) (Z) -2-hexen-6-amine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is piperidine a stronger base than pyridine?
A) The lone pair of electrons in pyridine is part of the delocalized p system.
B) Aromatic compounds are always less basic than non-aromatic compounds.
C) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp hybrid orbital.
D) The lone pair of electrons in piperidine is in an sp3 hybrid orbital; the lone pair of electrons in pyridine is in an sp2 hybrid orbital.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound?
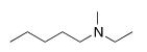
A) N-ethyl-N-methylpentylamine
B) N-ethyl-N-methyl-1-pentanamine
C) N-methyl-3-octanamine
D) N-ethyl-2-heptanamine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What starting materials are required to synthesize the following azo compound?
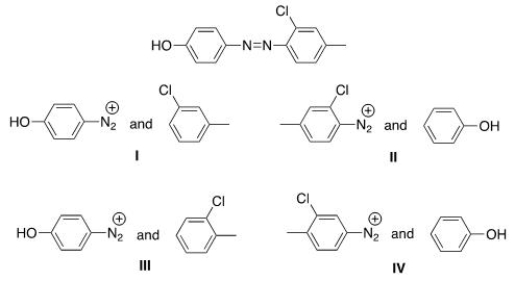
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectrum of an amine shows a parent peak with an odd mass for the molecular ion.What does this tell you about the amine?
A) The amine is a primary amine.
B) The amine is a secondary amine.
C) The amine contains an even number of N atoms.
D) The amine contains an odd number of N atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product obtained in the following reaction?
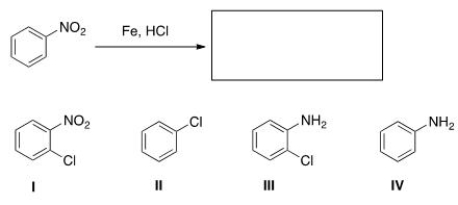
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 41 - 53 of 53
Related Exams