A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product (including stereochemistry) is formed in the following intermolecular reaction?
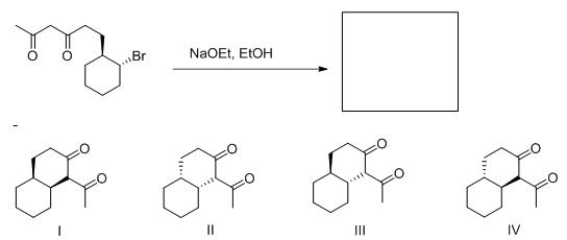
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the unsaturated carbonyl compound formed by the dehydration of the following b-hydroxy carbonyl compound?
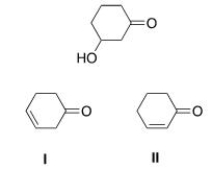
A) I
B) II
C) I and II
D) None of the choices
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a Michael reaction, where does the nucleophile attack the a,b-unsaturated carbonyl component?
A) a-Carbon
B) b-Carbon
C) Carbonyl carbon
D) Carbonyl carbon and b-carbon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction is an example of what type of reaction?
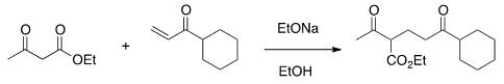
A) Michael reaction
B) Aldol self-condensation
C) Dieckmann condensation
D) Robinson annulation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name given to the Claisen reaction between two different esters?
A) Multiple Claisen reaction
B) Differential Claisen reaction
C) Crossed Claisen reaction
D) Versatile Claisen reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a Michael reaction, what is the name given to the a,b-unsaturated carbonyl component?
A) Michael donor
B) Michael enolate
C) Michael nucleophile
D) Michael acceptor
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the general name for the reaction product formed in an aldol addition reaction?
A) a-Hydroxy carbonyl compound
B) b-Hydroxy carbonyl compound
C) g-Hydroxy carbonyl compound
D) a,b-Hydroxy carbonyl compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the two starting materials for a Robinson annulation?
A) an a,b-Unsaturated carbonyl compound and an enolate
B) a b-Ketoester and an enolate
C) a 1,5-Dicarbonyl compound and an enolate
D) a 1,3-Dicarbonyl compound and an enolate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbonyl compounds does not undergo Aldol addition reactions when treated with aqueous sodium hydroxide?
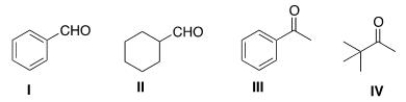
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a Claisen condensation reaction is run using methyl propanoate as the reactant, NaOCH3 is the ideal base.Why is it important to use NaOCH3 and not NaOCH2CH3?
A) NaOCH3 is a stronger base than NaOCH2CH3, and this reaction requires a stronger base.
B) NaOCH3 is a weaker base than NaOCH2CH3, and this reaction requires a weaker base.
C) Transesterfication can occur when esters react, and this transesterfication would result in a mixture of products.
D) NaOCH3 is more soluble than NaOCH2CH3 in organic solvents, and this reaction requires a full equivalent of base to proceed.A full equivalent of NaOCH2CH3 would not dissolve, so the reaction would not proceed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the general name for the reaction product formed in a Claisen reaction?
A) b-Hydroxy ester
B) b-Keto ester
C) a-Keto ester
D) g-Hydroxy ester
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following Claisen reaction?
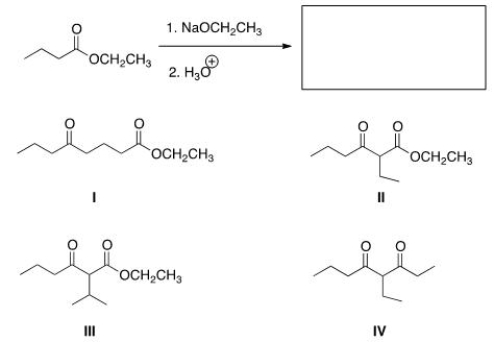
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In what situation can the yield of a single crossed Aldol product be increased?
A) The electrophilic carbonyl component is relatively unhindered and is used in excess.
B) The electrophilic carbonyl carbon component is relatively hindered and is used in limited amount.
C) The nucleophilic carbonyl component is relatively unhindered and is used in excess.
D) The nucleophilic carbonyl component is relatively hindered and is used in limited amount.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reaction type is an Aldol reaction?
A) Nucleophilic substitution
B) Electrophilic substitution
C) Electrophilic addition
D) Nucleophilic addition
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the self-condensation of ethanal (acetaldehyde) , shown below?
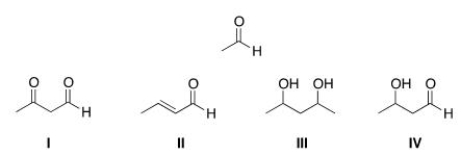
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the general name of the product of a crossed Claisen reaction between a ketone and an ester?
A) b-Keto ester
B) a,b-Dicarbonyl compound
C) g-Dicarbonyl compound
D) b-Dicarbonyl compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of esters can undergo Claisen reactions?
A) All esters can undergo Claisen reactions.
B) Only esters with two hydrogen atoms on the acarbon can undergo Claisen reactions.
C) Only esters with three hydrogen atoms on the acarbon can undergo Claisen reactions.
D) Only esters with two or three hydrogen atoms on the acarbon can undergo Claisen reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds can undergo an Aldol with itself?
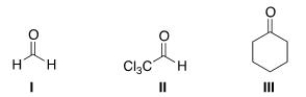
A) I
B) II
C) III
D) Both I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product (including stereochemistry) is formed in the following intermolecular reaction?

A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 45
Related Exams