A) Brønsted-Lowry acid and Lewis acid
B) Brønsted-Lowry base and Lewis base
C) Brønsted-Lowry base
D) Lewis base
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following concepts can be used to explain the difference in acidity between acetylene (C2H2) and ethylene (C2H4) ?
A) Size
B) Resonance
C) Inductive effect
D) Hybridization
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing acidity, putting the most acidic first. 
A) I > II > III
B) III > II > I
C) II > III > I
D) III > I > II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is the conjugate acid of ammonia, NH3?
A) H4N
B) H3N+
C) H2N-
D) H4N+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about a Brønsted-Lowry base is true?
A) The net charge may be zero, positive, or negative.
B) All Brønsted-Lowry bases contain a lone pair of electrons or a π bond.
C) All Brønsted-Lowry bases contain a proton.
D) The net charge may be zero or positive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A) CH3OH
B) H2O
C) CH3COOH
D) BF3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is the conjugate base of the hydronium ion, H3O+?
A) H3O
B) H2O-
C) H2O
D) HO-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following conjugate bases in order of decreasing basicity, putting the most basic first. 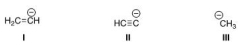
A) II > I > III
B) I > II > III
C) III > I > II
D) III > II > I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species is the conjugate base of methanol, CH3OH?
A) CH3OH2+
B) CH3O-
C) CH3-
D) CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is the weakest acid?
A) H2S
B) PH3
C) HCl
D) SiH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of decreasing acidity, putting the most acidic first. 
A) IV > II > III > I
B) IV > III > II > I
C) III > IV > II > I
D) III > IV > I > II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the Lewis base in the following reaction. 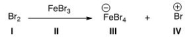
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the Lewis acid in the following reaction. 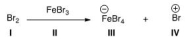
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about acid strength is true?
A) The stronger the acid, the further the equilibrium lies to the left.
B) The stronger the acid, the smaller the Ka.
C) The stronger the acid, the larger the pKa.
D) The stronger the acid, the smaller the pKa.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements explain why HBr is a stronger acid than HF?
A) Br- is more stable than F- because Br- is larger than F-.
B) Br- is less stable than F- because Br- is larger than F-.
C) Br- is more stable than F- because Br- is less electronegative than F-.
D) Br- is less stable than F- because Br- is less electronegative than F-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is a correct definition for a Brønsted-Lowry acid?
A) Proton acceptor
B) Electron pair donor
C) Electron pair acceptor
D) Proton donor
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the conjugate base in the following reaction?

A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the strongest base?
A) CH3COCH3
B) CH3COOH
C) NH3
D) H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about Lewis acids is true?
A) Lewis acids are proton donors.
B) Lewis acids are proton acceptors.
C) Lewis acids are electron pair donors.
D) Lewis acids are electron pair acceptors.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following compounds in order of increasing acidity, putting the least acidic first. 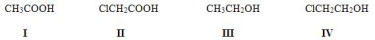
A) III < I < IV < II
B) III < IV < I < II
C) II < I < IV < III
D) III < I < II < IV
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 52
Related Exams