A) An absorption at 3100 cm-1
B) A signal at 113.
C) A signal at 112 and a signal at 114 in a ratio of 3:1
D) A signal at 112 and a signal at 114 in a ratio of 1:1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Examine the IR below and classify the compound. 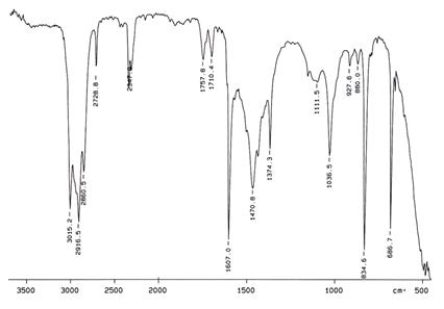
A) Alcohol
B) Arene
C) Amine
D) Ketone
E) Carbocylic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of signal(s) would you observe in the mass and (or) infrared spectrum of the following compound?
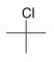
A) A signal at 1600 cm-1
B) A signal at 3300 cm-1
C) A single mass peak at 92 amu
D) Two mass peaks at 92 and 94 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 94 and shows prominent peaks in its IR spectrum at 3600-3200 and 1600 cm-1?
A) The compound has a molecular mass of 94.
B) The compound contains a C=O group and Csp3-H hybridized bonds.
C) The compound contains an OH group and a benzene ring.
D) Both A (The compound has a molecular mass of 94) and B (The compound contains a C=O group and Csp3-H hybridized bonds) are true statements.
E) Both A (The compound has a molecular mass of 94) and C (The compound contains an OH group and a benzene ring) are true statements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the infrared absorption for the stretching motion of internal alkynes rarely observed?
A) They do not form cations.
B) They are too strong.
C) Stretching in internal alkynes does not involve a change in dipole moment.
D) They do not have hydrogens.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Examine the IR below and classify the compound. 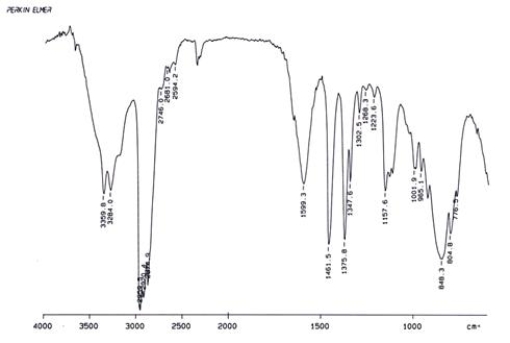
A) Alcohol
B) Aldehyde
C) Amine
D) Ketone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An IR spectrum has the following potentially important absorptions: 3091, 3067, 2963, 2921, 2252, 1603, 1499, 1455, 1416, 1078, 1031, 941, 735, and 696 cm-1.Indicate which structure corresponds to the IR data. 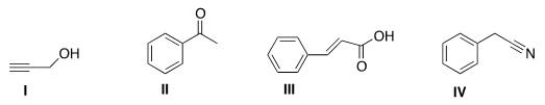
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following π bonds is IR inactive?
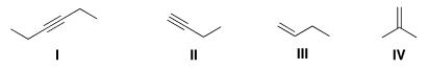
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You observe a compound that exhibits a mass spectrum with peak at 160 and a peak at 162, both of equal intensity.This compound contains:
A) Two chlorine atoms
B) One iodine atom
C) One bromine atom
D) Two bromine atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statement(s) is (are) true about a compound that has a molecular ion peak in its mass spectrum at mass 69 and shows a prominent peak in its IR spectrum at 2250 cm-1?
A) The compound has a molecular mass of 70.
B) The compound contains a C=O group.
C) The compound contains a cyano or alkyne group.
D) Both (The compound has a molecular mass of 70) and (The compound contains a C=O group) are true statements.
E) Both (The compound has a molecular mass of 70) and (The compound contains a cyano or alkyne group) are true statements.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The base peak in a mass spectrum corresponds to the most stable fragment.Propose a structure for a compound that is consistent with the following data.a) The molecular ion peak has m/z = 116.b) The base peak is at m/z = 59.c) The compound is composed of C, H and O atoms.d) The IR spectrum shows a strong absorbance at 3257 cm-1. 
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the phenol shown below is treated with KOH, it forms a product whose IR spectrum does not show an absorption in the 3200-3600 cm-1 region.Propose a structure for the product. 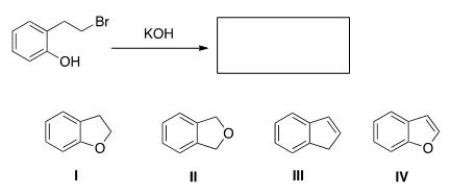
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the molecular formula of rose oxide which contains C, H, and O and has two degrees of unsaturation and a molecular ion in its mass spectrum at m/z =154?
A) C10H18O
B) C9H14O2
C) C10H2O2
D) C8H10O3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a typical mass spectrum, a smaller signal is observed at a mass 1 amu higher than the molecular ion peak.Why?
A) Due to small impurities in the sample
B) Machine error
C) Because a small percentage of the compound will have a carbon that is the isotope 13C instead of 12C
D) Because of fragmentation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In electron impact mass spectrometry (EIMS) , what is being detected?
A) The molecular mass of the compound
B) The molecular formula of the compound
C) The mass to charge ratio of any ionic species
D) The mass to charge ratio of any neutral species
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following structures is consistent with a compound that displays a molecular ion peak at 103 and infrared signals at 2250 and 1600 cm-1?
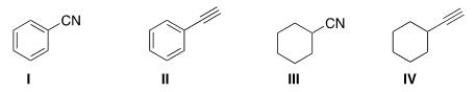
A) I
B) II
C) III
D) IV
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Examine the IR below and classify the compound. 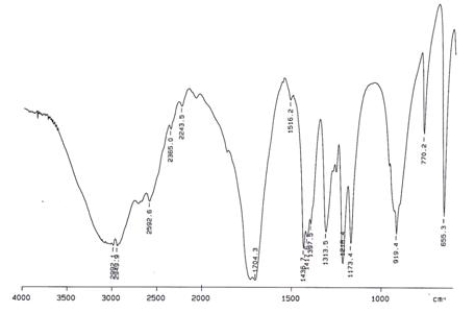
A) Alcohol
B) Aldehyde
C) Carboxylic acid
D) Ketone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is consistent with a compound that displays a molecular ion peak at 84 and infrared signals at 3000-2850 cm-1 and no signals between 3000-3300 cm-1?
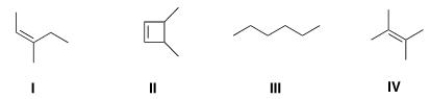
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the base peak of a mass spectrum is always true?
A) The base peak corresponds to the molecular ion.
B) The base peak corresponds to the most abundant ion.
C) The base peak corresponds to the lowest m/z.
D) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is consistent with a compound that displays a molecular ion peak at 56 and infrared signals at 2250 and 3600-3200 cm-1?
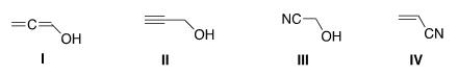
A) I
B) II
C) III
D) IV
Correct Answer

verified
B
Correct Answer
verified
Showing 1 - 20 of 35
Related Exams