A) Yes.K2CO3(aq) + Ca(NO3) 2(aq) KNO3(aq) + CaK(s)
B) No reaction will occur.
C) Yes.K2CO3(aq) + Ca(NO3) 2(aq) KNO3(aq) + CaCO3(s)
D) Yes.K2CO3(aq) + Ca(NO3) 2(aq) K2(NO3) 2(aq) + CaCO3(s)
E) Yes.K2CO3(aq) + Ca(NO3) 2(aq) 2KNO3(aq) + CaCO3(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the figure shown, is a chemical reaction occurring? 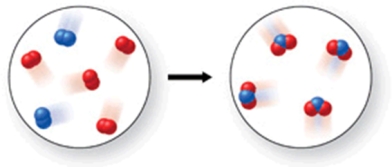
A) No, because there are the same number of atoms in both images.
B) Yes, because the atoms have rearranged, and therefore the formulas of the products and reactants are different.
C) No, because there are oxygen atoms and nitrogen atoms in both images.
D) No, because both the reactants and products are colorless gases.
E) Yes, because the reactants are gases, but the product is a solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When aqueous solutions of NaOH and MgCl2 are mixed, a precipitate forms.What is the correct formula for the precipitate?
A) Mg(OH) 2(s)
B) NaCl(s)
C) NaCl2(s)
D) MgOH(s)
E) MgCl2(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic compounds would be expected to be insoluble in water?
A) NaOH
B) KI
C) CaS
D) Ca(NO3) 2
E) Na2SO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The class of the reaction shown in the figure is: 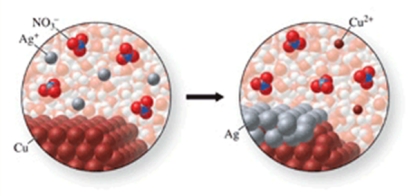
A) combination reaction.
B) decomposition reaction.
C) double-displacement reaction.
D) single-displacement reaction.
E) combustion reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction which has one element and one compound as reactants and one element and one compound as products is:
A) a double-displacement reaction.
B) a combination reaction.
C) a decomposition reaction.
D) a combustion reaction.
E) a single-displacement reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the following skeletal equation: C3H8(g) + O2(g) CO2(g) + H2O(g) .
A) C3H8(g) + O2(g) 3CO2(g) + 4H2O(g)
B) C3H8(g) + O2(g) 3CO2(g) + 2H2O(g)
C) C3H8(g) + 3O2(g) 3CO2(g) + 4H2O(g)
D) C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
E) C3H8(g) + 4O2(g) 3CO2(g) + 4H2O(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The class of the reaction shown in the figure is: 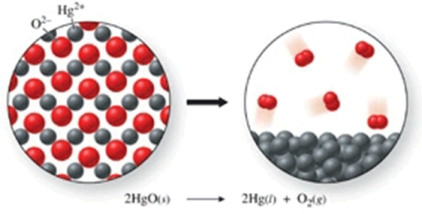
A) double-displacement reaction.
B) combination reaction.
C) combustion reaction.
D) decomposition reaction.
E) single-displacement reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Would an aqueous solution of CH3OH contain ions? If so, indicate the formulas of the ions in the solution.
A) Yes.CH4+(aq) + Oˉ(aq)
B) Yes.CH3ˉ(aq) + OH+(aq)
C) Yes.CH3+(aq) + OHˉ(aq)
D) Yes.CH42+(aq) + O2ˉ(aq)
E) No, this substance would not form ions in solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a balanced equation to represent the reaction shown in the figure. 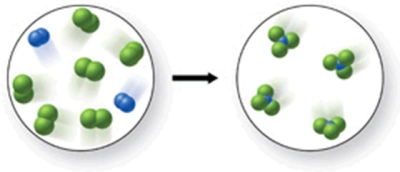
A) 2N2 + 6Cl2 3NCl4
B) N2 + Cl2 2NCl3
C) 2N2 + 6Cl2 4NCl3
D) N2 + 6Cl2 4NCl3
E) 2N2 + 5Cl2 3NCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction which has two compounds as reactants and two compounds as products is:
A) a combustion reaction.
B) a single-displacement reaction.
C) a decomposition reaction.
D) a combination reaction.
E) a double-displacement reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify which image in the figure represents the reactants and which image therefore represents the products in the reaction between ozone gas (O3) and carbon monoxide gas to form oxygen gas and carbon dioxide gas. 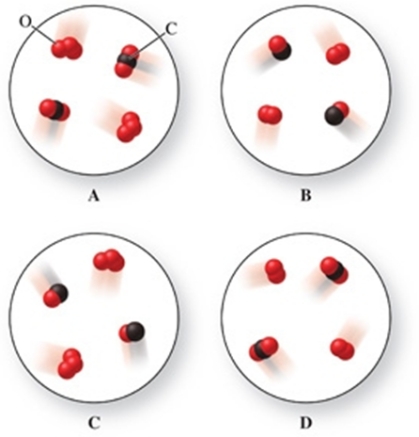
A) upper left image = reactants, upper right image = products
B) upper right image = reactants, upper left image = products
C) lower left image = reactants, lower right image = products
D) upper left image = reactants, lower right image = products
E) upper left image = reactants, lower left image = products
Correct Answer

verified
Correct Answer
verified
True/False
The compound PbCl2 would be expected to be water-soluble.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the figure shown, is a chemical reaction occurring? 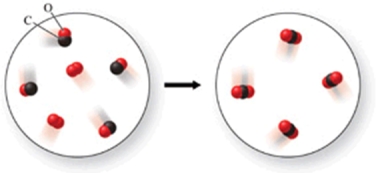
A) No, because there are the same number of atoms in both images.
B) Yes, because the atoms have rearranged, and therefore the formulas of the products and reactants are different.
C) No, because both the reactants and products are colorless gases.
D) No, because there are oxygen atoms and carbon atoms in both images.
E) Yes, because the reactants are gases, but the product is a solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction which has two elements as reactants and one compound as a product is:
A) a single-displacement reaction.
B) a decomposition reaction.
C) a combustion reaction.
D) a double-displacement reaction.
E) a combination reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the combination reaction that occurs when magnesium metal reactions with oxygen gas?
A) MgO2(s)
B) Mg2O3(s)
C) Mg2O(s)
D) MgO(s)
E) Mg2O2(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When aqueous solutions of potassium chloride and lead(II) nitrate are mixed, a double-displacement reaction occurs.What is the balanced equation for the reaction?
A) KCl(aq) + Pb2NO3(aq) KNO3(aq) + PbCl(aq)
B) KCl(aq) + Pb(NO3) 2(aq) K(s) + PbNO3Cl(aq)
C) KCl(aq) + Pb2NO3(aq) KNO3(aq) + 2PbCl(s)
D) 2KCl(aq) + Pb(NO3) 2(aq) KNO3(aq) + ClPb(s)
E) 2KCl(aq) + Pb(NO3) 2(aq) 2KNO3(aq) + PbCl2(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When zinc metal is placed into a copper(II) nitrate solution, a single-displacement reaction occurs.Write a balanced equation to describe this reaction.
A) 2Zn(s) + Cu(NO3) 2(aq) 2Zn(NO3) 2(aq) + Cu(s)
B) Zn(s) + Cu(NO3) 2(aq) ZnNO3(aq) + CuNO3(aq)
C) Zn(s) + Cu2NO3(aq) ZnNO3(aq) + Cu(aq)
D) Zn(s) + Cu(NO3) 2(aq) Zn(NO3) 2(aq) + Cu(s)
E) Zn(s) + Cu2NO3(aq) ZnNO3(aq) + 2Cu(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following changes represents a physical change, rather than evidence of a chemical reaction?
A) When water and a yellow solution of antifreeze are mixed, the resulting solution is a lighter shade of yellow than the original antifreeze solution.
B) When concentrated HNO3 is placed in contact with copper metal, a brown gas is formed, the copper dissolves, and a green solution is formed.
C) When an egg is fried, the clear part becomes white, and the egg becomes a solid.
D) When a piece of zinc metal is placed in a blue solution of copper sulfate, the solution turns from blue to colorless, the zinc dissolves, and copper metal is formed.
E) When solutions of AgNO3 and NaCl are mixed, a white solid forms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The class of the reaction shown in the figure is: 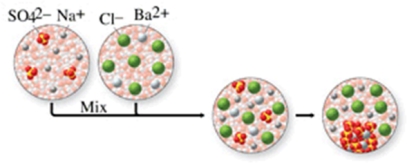
A) combination reaction.
B) combustion reaction.
C) single-displacement reaction.
D) double-displacement reaction.
E) decomposition reaction.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 108
Related Exams