A) C2O32-
B) CO32-
C) HCO32-
D) HCO3 -
E) C2O42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following combinations of elements in a compound would be ionic?
A) barium and fluorine
B) hydrogen and sulfur
C) sulfur and oxygen
D) hydrogen and fluorine
E) all of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the boiling points of the following compounds from lowest to highest: C12H22O11 (sucrose) , O2, NaF
A) C12H22O11 (sucrose) < O2 < NaF
B) O2 < NaF < C12H22O11 (sucrose)
C) O2 < C12H22O11 (sucrose) < NaF
D) NaF < O2 < C12H22O11 (sucrose)
E) NaF < C12H22O11 (sucrose) < O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would you expect to exist as ions when dissolved in water? KNO3, HBr, CH3OH
A) KNO3 only
B) HBr only
C) CH3OH only
D) KNO3 and HBr
E) HCl and CH3OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formula for dinitrogen pentoxide is:
A) NO5
B) N2O4
C) N2O5
D) NO6
E) N2O6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination of formula and name is incorrect?
A) Na+ = sodium ion
B) Ca2+ = calcium ion
C) N2- = nitride ion
D) F- = fluoride ion
E) O2-= oxide ion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the substance(s) shown in the figure is molecular? 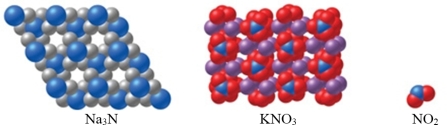
A) Na3N
B) KNO3
C) NO2
D) Both Na3N and KNO3
E) Both KNO3 and NO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following combinations of formula and name is incorrect?
A) nitride ion = NO2-
B) cyanide ion = CN-
C) chlorite ion = ClO2-
D) perchlorate ion = ClO4-
E) chloride ion = Cl-
Correct Answer

verified
Correct Answer
verified
True/False
SO3 and SO32-have the same number of each type of atom, so they have the same name and the same chemical properties.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compound names is not correct?
A) iron(III) sulfate
B) copper(II) diphosphide
C) sodium hydroxide
D) potassium nitrate
E) calcium sulfide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for the compound formed between the calcium ion and the sulfate ion?
A) CaSO4
B) Ca2(SO4) 2
C) Ca(SO4) 2
D) CaS
E) CaSO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following combinations of formula and name is incorrect?
A) carbonate ion = CO32-
B) ammonium ion = NH3
C) bicarbonate ion = HCO3-
D) periodate ion = IO4-
E) hypochlorite ion = ClO-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of ions is formed when (NH4) 2SO4 dissolves in water?
A) (NH4) 22+ + SO42-
B) 2NH3 + 2H+ +SO42-
C) 2NH4 + + SO42-
D) 2N3- + 8H+ + S+6 + 4O2-
E) 2N3- + 8H+ + SO42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the oxoanion of nitrogen with a 1-charge shown in the figure is: 
A) nitrooxate ion.
B) nitride ion.
C) nitrite ion.
D) nitrate ion.
E) nitrous ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes HCl when dissolved in water? 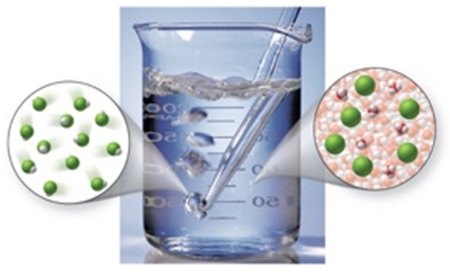
A) strong electrolyte
B) weak electrolyte
C) nonelectrolyte
D) exists as atoms and molecules
E) exists as molecules only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for the iodate ion is IO3-.What is the formula for the hypoiodite ion?
A) IO4-
B) IO2-
C) IO-
D) IO32-
E) I-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formulas for a compound containing the Fe2+ ion is incorrect?
A) FeO
B) FeCl
C) FeS
D) FeSO4
E) Fe(NO3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for the chlorite ion is:
A) ClO-
B) ClO2-
C) ClO3-
D) ClO4-
E) ClO22-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the oxoanion of nitrogen with a 1- charge shown in the figure is: 
A) nitrooxate ion.
B) nitride ion.
C) nitrite ion.
D) nitrate ion.
E) nitrous ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is classified as an ionic compound?
A) NO2
B) CF4
C) CH3OH
D) CaCO3
E) all of these
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 112
Related Exams