Correct Answer

verified
Correct Answer
verified
Essay
Starting from benzene, devise two multistep syntheses to make the compound shown here. 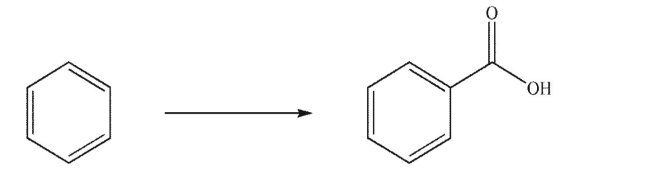
Correct Answer

verified
Correct Answer
verified
Essay
Isophthaloyl chloride was used as a starting material in a synthesis of the following polymer.What
was the structure of its coupling partner X? 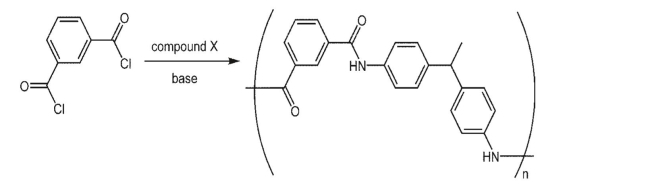
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true?
A) Carboxylate anions are more susceptible to nucleophilic addition than carboxylic acids.
B) Polarization in the C=O bond destabilizes a carboxylate anion.
C) The carbonyl carbon atom in a carboxylic acid reacts as a Lewis base.
D) Protonation of a carboxylic acid generally occurs faster at the hydroxyl group than at the carbonyl oxygen.
E) Removal of the hydroxyl proton in a carboxylic acid is often the fastest reaction that carboxylic acids undergo.
Correct Answer

verified
Correct Answer
verified
Essay
Heating of the lactam shown below results in a linear polyamide.Draw its structure. 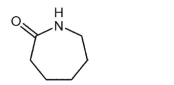
Correct Answer

verified
11eb4b6a_e18d_91af_80d3_4dda02d7d7dc_TB4310_00
Correct Answer
verified
Multiple Choice
What is reagent X? 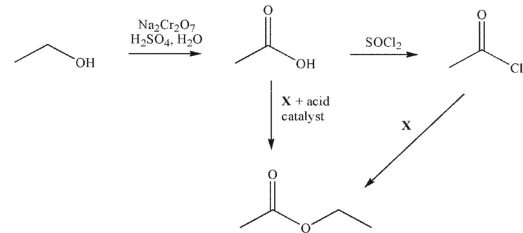
A) CH3CH2Br
B) NaBH4
C) OsO4
D) ethanol
E) H3O+
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the product of the following sequence of reactions? 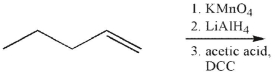
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
What is the product of the following reaction? 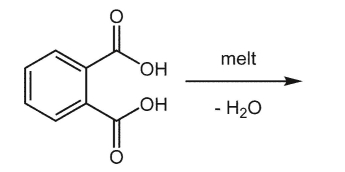
Correct Answer

verified
11eb4b6a_e18d_dfd7_80d3_8bc7205766fa_TB4310_00
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 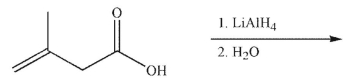
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Predict the major organic product of the following reaction conditions. 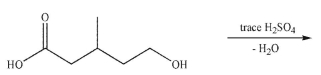
Correct Answer

verified
Correct Answer
verified
Essay
Design a multistep synthesis of the target molecule from the starting material shown.Show the reagents needed for each step and the product of each step. 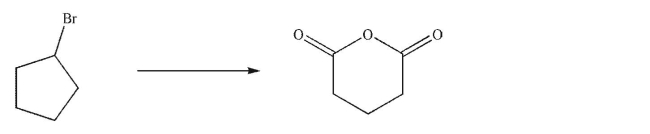
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reagent would you use to accomplish the following transformation? 
A) LiAlH4
B) CH3Br, then H3O+
C) 1 equiv. CH3Li, then H3O+
D) 2 equiv. CH3Li, then H3O+
E) CH3OH and trace H2SO4
Correct Answer

verified
Correct Answer
verified
Essay
In the presence of a strong acid, one of the oxygen atoms on the carboxylic acid shown here will become protonated.Which oxygen atom is protonated and why? 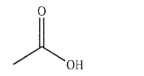
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major organic product of the following reaction.
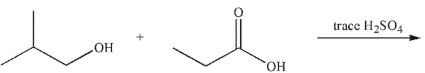
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these carboxylic acids is in the s-cis conformation?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) both c and d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct name for the compound shown here?

A) 2-methyl butanoic acid
B) 2-ethyl propanoic acid
C) (S) -2 -methyl butanoic acid
D) (R) -2 -methyl butanoic acid
E) (S) -2 -ethyl propanoic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reagents could be used to accomplish the following transformation?

A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) either a or b
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the following reaction? 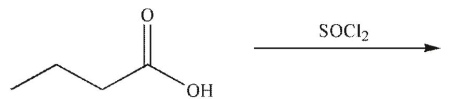
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) No reaction would occur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these structures is not a mechanistic intermediate in acid-catalyzed esterification of a carboxylic acid?
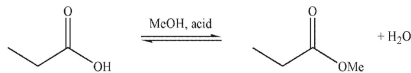
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Design a multistep synthesis of the target molecule from the starting material shown.Show the reagents needed for each step and the product of each step. 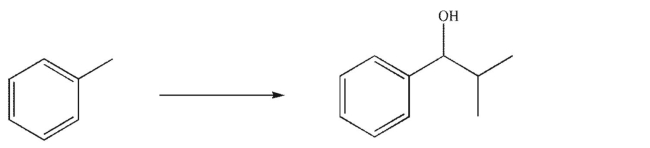
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 66
Related Exams