A) 10 torr
B) 50 torr
C) 100 torr
D) 200 torr
E) 1000 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dr. I. M. A. Brightguy adds 0.1727 g of an unknown gas to a 125-mL flask. If Dr. B finds the pressure to be 736 torr at 20.0°C, is the gas likely to be methane, CH4, nitrogen, N2, oxygen, O2, neon, Ne, or argon, Ar?
A) CH 4
B) N 2
C) Ne
D) Ar
E) O 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases will be the slowest to diffuse through a room?
A) Methane, CH 4
B) Hydrogen sulfide, H 2S
C) Carbon dioxide, CO 2
D) Water, H 2O
E) Neon, Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of methane gas, CH4(g) , occupies a volume of 60.3 L at a pressure of 469 torr and a temperature of 29.3°C. What would be its temperature at a pressure of 243 torr and volume of 60.3 L?
A) −116.5°C
B) 15.2°C
C) 15.5°C
D) 57.7°C
E) 310.6°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 750-mL sample of hydrogen exerts a pressure of 822 torr at 325 K. What pressure does it exert if the temperature is raised to 475 K at constant volume?
A) 188 torr
B) 562 torr
C) 1.11 × 10 3 torr
D) 1.20 × 10 3 torr
E) 1.90 × 10 3 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the molecular mass of a gas increases by a factor of 4 at constant temperature, its rms speed will
A) decrease by a factor of 4.
B) increase by a factor of 4.
C) decrease by a factor of 16.
D) increase by a factor of 16.
E) decrease by a factor of 2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 3.0-L sample of helium was placed in a container fitted with a porous membrane. Half of the helium effused through the membrane in 24 hours. A 3.0-L sample of oxygen was placed in an identical container. How many hours will it take for half of the oxygen to effuse through the membrane?
A) 8.5 h
B) 12 h
C) 48 h
D) 60. h
E) 68 h
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen dioxide is a red-brown gas that is responsible for the color of photochemical smog. A sample of nitrogen dioxide has a volume of 28.6 L at 45.3°C and 89.9 kPa. What is its volume at STP?
A) 21.8 L
B) 27.6 L
C) 29.6 L
D) 37.6 L
E) 153 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask containing argon gas is connected to a closed-ended mercury manometer. The closed end is under vacuum. If the mercury level in the closed arm is 230. mm above that in the arm connected to the flask, what is the argon pressure, in torr?
A) −230.
B) 230.
C) 530.
D) 790.
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Magnesium metal (0.100 mol) and a volume of aqueous hydrochloric acid that contains 0.500 mol of HCl are combined and react to completion. How many liters of hydrogen gas, measured at STP, are produced? Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
A) 2.24 L of H 2
B) 4.48 L of H 2
C) 5.60 L of H 2
D) 11.2 L of H 2
E) 22.4 L of H 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a single molecule of water is 2.99 × 10−23 mL. For a sample of gaseous water at 1.00 atm and 150°C, what fraction of the container's volume is occupied by the molecules themselves?
A) 5.2 × 10 −7
B) 4.5 × 10 −5
C) 5.2 × 10 −4
D) 5.2 × 10 −1
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm. What is the molar mass of the gas?
A) 7.76 g/mol
B) 66.1 g/mol
C) 74.0 g/mol
D) 81.4 g/mol
E) 144 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assuming ideal behavior, what is the density of argon gas at STP, in g/L?
A) 0.0176 g/L
B) 0.0250 g/L
C) 0.0561 g/L
D) 1.78 g/L
E) 181. g/L
Correct Answer

verified
Correct Answer
verified
True/False
According to the postulates of kinetic-molecular theory, the molecules of all gases at a given temperature have the same average speed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen gas exerts a pressure of 466 torr in a container. What is this pressure in atmospheres?
A) 0.217 atm
B) 0.466 atm
C) 0.613 atm
D) 1.63 atm
E) 4.60 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the lines on the figure below is the best representation of the relationship between the volume and the number of moles of a gas, measured at constant temperature and pressure? 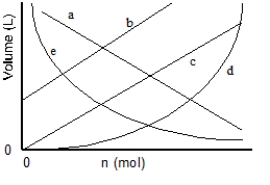
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask containing neon gas is connected to an open-ended mercury manometer. The open end is exposed to the atmosphere, where the prevailing pressure is 745 torr. The mercury level in the open arm is 50. mm below that in the arm connected to the flask of neon. What is the neon pressure, in torr?
A) −50. torr
B) 50. torr
C) 695 torr
D) 795 torr
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 250.0-mL sample of ammonia, NH3(g) , exerts a pressure of 833 torr at 42.4°C. What mass of ammonia is in the container?
A) 0.0787 g
B) 0.180 g
C) 8.04 g
D) 17.0 g
E) 59.8 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"The pressure of an ideal gas is inversely proportional to its volume at constant temperature and number of moles" is a statement of __________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen peroxide was catalytically decomposed and 75.3 mL of oxygen gas was collected over water at 25°C and 742 torr. What mass of oxygen was collected? (Pwater = 24 torr at 25°C)
A) 0.00291 g
B) 0.0931 g
C) 0.0962 g
D) 0.0993 g
E) 0.962 g
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 98
Related Exams