A) carbonate ion = CO32-
B) ammonium ion = NH3
C) bicarbonate ion = HCO3-
D) periodate ion = IO4-
E) hypochlorite ion = ClO-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formulas for the compounds in the figure are: 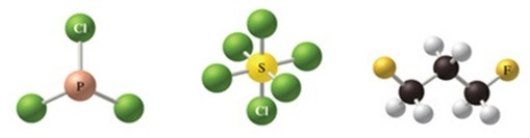
A) Cl3P, Cl6S, C3H2F6
B) PCl3, SCl6, C3H6F2
C) PCl3, SCl4, C3H6F3
D) P3Cl, S6Cl, C3H6F2
E) PCl3, SCl8, C3H6F2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for the compound formed between iron(III) ion and the oxide ion?
A) FeO
B) Fe2O3
C) Fe3O2
D) Fe3O4
E) FeO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name combinations is incorrect?
A) N2O4 dinitrogen tetroxide
B) ClO2 dichlorine oxide
C) SO3 sulfur trioxide
D) CS2 carbon disulfide
E) PCl3 phosphorus trichloride
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formulas for a compound containing the Cr3+ ion is incorrect?
A) Cr(OH) 3
B) Cr2(SO4) 3
C) CrN
D) CrNO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the compound with the name aluminum sulfide?
A) AlS
B) Al3S2
C) Al2S3
D) AlSO4
E) Al2(SO4) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the bromide ion?
A) Br2
B) Br2-
C) Br22-
D) Br-
E) BrO3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name combinations is incorrect?
A) CS2 carbon disulfide
B) SO2 sulfur dioxide
C) N2O5 dinitrogen tetroxide
D) PF3 phosphorus trifluoride
E) P4O10 tetraphosphorus decoxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formulas for the compounds in the figure are: 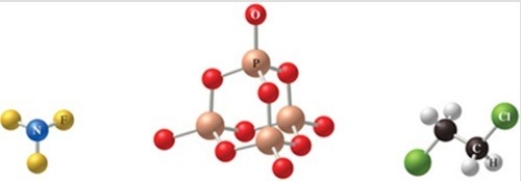
A) NF3, P4O10, C2H4Cl2
B) NF3, P4O8, C2H4Cl2
C) NF3, P4O10, C2H2Cl4
D) NF4, P3O10, C2H2Cl4
E) NF4, P3O10, C2H2Cl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compound names is not correct?
A) calcium hydroxide
B) iron(II) dichloride
C) potassium fluoride
D) sodium cyanide
E) ammonium hydroxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct formula for dinitrogen pentoxide is:
A) NO5
B) N2O4
C) N2O5
D) NO6
E) N2O6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct name for the compound Mn2O3?
A) dimanganese trioxide
B) manganate ion
C) manganese(III) oxide
D) manganese(II) oxide
E) manganese oxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements regarding ionic and molecular compounds is correct?
A) Molecular compounds are expected to have very high melting and boiling points compared to ionic compounds.
B) Molecular compounds are strong electrolytes in aqueous solution.
C) An ionic compound can be a gas, liquid, or solid at room temperature.
D) Molecular compounds form crystalline solids that are hard and brittle.
E) Ionic compounds are expected to have very high melting and boiling points compared to ionic compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aluminum sulfate, calcium oxide, and water react together in a process used to remove solids from treated water. Which set of formulas correspond to these compounds?
A) AlSO4, Ca2O, H2O
B) Al3(SO4) 2, Ca2O, H2O
C) Al2(SO4) 3, Ca2O, H2O2
D) Al2(SO4) 3, CaO2, H2O2
E) Al2(SO4) 3, CaO, H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name combinations is incorrect?
A) HNO2(aq) nitrous acid
B) HBr(aq) hydrobromic acid
C) H2SO3(aq) sulfurous acid
D) HClO2(aq) hypochlorous acid
E) H3PO4(aq) phosphoric acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds should be crystalline, brittle solids at room temperature and are electrolytes? MgCl2, CCl4, N2O4, NaOH
A) MgCl2 and CCl4
B) N2O4 only
C) N2O4 and NaOH
D) NaOH only
E) MgCl2 and NaOH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is formed when (NH4) 2SO4 dissolves in water?
A) (NH4) 22+ + SO42-
B) 2NH3 + 2H+ +SO42-
C) 2NH4+ + SO42-
D) 2N3- + 8H+ + S+6 + 4O2-
E) 2N3- + 8H+ + SO42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the compound with the name iron(II) phosphate?
A) FeP
B) Fe3(PO4) 2
C) FePO4
D) Fe2(PO3) 3
E) Fe2(PO4) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the substances shown in the figure is a molecular compound? 
A) Na3N
B) KNO3
C) NO2
D) Both Na3N and KNO3
E) Both KNO3 and NO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name combinations is incorrect?
A) SF6 sulfur hexafluoride
B) NO2 nitrogen dioxide
C) PF5 phosphorus pentafluoride
D) BN boron nitride
E) ICl7 iodine hexachloride
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 113
Related Exams