A) 3-methylbutene
B) 1-butene
C) 2-butene
D) 2-methylpropene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true of London dispersion forces?
A) London dispersion forces are stronger between the molecules of 1-butanol than between the molecules of 2-methyl-2-propanol.
B) London dispersion forces are weaker than hydrogen bonding interactions.
C) London dispersion forces are stronger between the molecules of 1-butanol than between the molecules of 2-methyl-2-propanol and are weaker than hydrogen bonding interactions.
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct order of the boiling points of propane, dimethyl ether, and ethanol?
A) ethanol > dimethyl ether > propane
B) ethanol > propane > dimethyl ether
C) propane > dimethyl ether > ethanol
D) propane > ethanol > dimethyl ether
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes the products formed by the dehydration of 2,2,4,4-tetramethyl-3-pentanol?
A) The only product formed is 2,2,4,4-tetramethyl-2-pentene.
B) The only product formed is 2,2,4,4-tetramethyl-3-pentene.
C) The major product is 2,2,4,4-tetramethyl-2-pentene, and the minor product is 2,2,4,4-tetramethyl-3-pentene.
D) There is no product formed because 2,2,4,4-tetramethyl-3-pentanol does not undergo dehydration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the smallest number of carbon atoms that can be found in an asymmetrical ether containing only C, H, and a single O atom?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following classes of compounds react with sodium hydroxide to give a water-soluble salt?
A) primary alcohols
B) phenols
C) both primary alcohols and phenols
D) neither primary alcohols nor phenols
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an important intermediate in the production of ethylene glycol?
A) diethyl ether
B) ethanol
C) ethylene oxide
D) tetrahydrofuran
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the common name of the following compound? 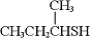
A) isobutyl mercaptan
B) sec-butyl mercaptan
C) 2-methyl-1-propanthiol
D) 2-methyl-1-propanethiol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thiols are most closely related to which of the following classes of compounds?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of hydrogen bonds that an ethanol molecule can form with other molecules?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify a true statement about trans-2-methylcyclohexanol.
A) The hydroxyl group and the methyl group lie in the plane that contains the cyclohexane ring.
B) The methyl group may lie above and the hydroxyl group may lie below the plane that contains the cyclohexane ring.
C) The methyl group and the hydroxyl group lie above the plane that contains the cyclohexane ring.
D) The methyl group and the hydroxyl group lie below the plane that contains the cyclohexane ring.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ethers is used as an inhalation anesthetic today?
A) ![]()
B) ![]()
C) both ![]() and
and![]()
D) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify a true statement about dimethyl ether.
A) It is a nonpolar compound.
B) Its boiling point is comparable to the boiling point of ethanol.
C) The carbon bonded to oxygen in a dimethyl ether molecule carries a partially positive charge.
D) Strong attractive forces exist between dimethyl ether molecules in the liquid form.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry at the hydroxyl-bearing carbon atom of an alcohol?
A) linear
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of alcohols and phenols?
A) Both have equal basic strength.
B) Both have equal acidic strength.
C) Phenols are more acidic than alcohols.
D) Alcohols are more acidic than phenols.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is true of the polarities of the C-O and O-H bonds in alcohols?
A) Neither the C-O nor the O-H bond is polar.
B) The C-O and O-H bonds are equally polar.
C) Both bonds are polar, but the C-O bond is less polar than the O-H bond.
D) Both bonds are polar, but the O-H bond is less polar than the C-O bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following can be obtained using propene as a starting material?
A) glycerin
B) isopropyl alcohol
C) epichlorohydrin
D) all of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the characteristic functional group of thiols?
A) -NH
B) -OH
C) -PH
D) -SH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
By which of the following reaction sequences is ethanol converted to ethanoic acid?
A) successive oxidation
B) successive reduction
C) oxidation followed by reduction
D) reduction followed by oxidation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a starting material in the synthesis of nitroglycerin?
A) ethylene glycol
B) 1,2-propanediol
C) 1,3-propanediol
D) 1,2,3-propanetriol
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 118
Related Exams