A) Pa
B) Bi
C) U
D) Ra
E) Rb
Correct Answer

verified
Correct Answer
verified
True/False
A beta particle is a proton.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What element is the stable end-product of the uranium radioactive decay series?
A) Th
B) Pu
C) Ra
D) Au
E) Pb
Correct Answer

verified
Correct Answer
verified
Short Answer
What technique would you use to determine how old a cloth is that was found while digging in Egypt?
Correct Answer

verified
Correct Answer
verified
True/False
Nuclear fusion is the combination of small nuclei into larger ones.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the relationship between the energy released (ΔE) and the mass defect (Δm) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total binding energy of 12C? [12C atomic mass = 12.000 amu; mass of proton = 1.007825 amu; mass of neutron = 1.008665 amu; 1 kg = 6.022 x 1026 amu; 1 J = 1 (kg•m2) /s2]?
A) 1.80 x 10-9 J
B) 1.48 x 10-8 J
C) 3.60 x 10-9 J
D) 8.91 x 1015 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is carbon dating possible?
A) The amount of C-12 is not variable in living organisms.
B) There is a constant ratio of C-12 to C-14 in living organisms but the ratio changes at a predictable rate after an organism dies.
C) The amount of C-14 is constant in living organisms.
D) None of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the missing symbol in this plutonium fission reaction? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many 14C atoms are in a charcoal sample that has a decay rate of 3500 disintegrations per min? (For 14C, t1/2 = 5730 yr.)
A) 2.9 × 107 atoms
B) 8.0 × 10-7 atoms
C) 1.4 × 1014 atoms
D) 1.5 × 1013 atoms
E) 6.02 × 1020 atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is used to image the liver?
A) (18O)
B) (131I)
C) (123I)
D) (24Na)
E) (99Tc)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 55-kg person exposed to thorium-234 receives 7.5 × 104 β particles, each with an energy of 1.6 × 10-14 J. How many rads does the person receive?
A) 2.1 × 10-19 rads
B) 1.2 × 10-17 rads
C) 2.2 × 10-9 rads
D) 1.2 × 10-9 rads
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction, identify X. 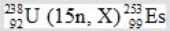
A) 7β
B) 3α
C) 4n
D) ![]()
E) 15p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following materials is put into a nuclear reactor to slow the chain reaction?
A) Heavy water
B) Moderators
C) Control rods
D) Reflectors
E) Chlorine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 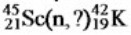
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
A plot of the number of neutrons versus the number of protons in various isotopes produces a "belt of stability." Isotopes below the belt of stability (i.e., with a smaller neutron-to-proton ratio) decay by beta particle emission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Petroleum is a fossil fuel containing many different carbon compounds. If the carbon atoms in petroleum have been in the ground for 100 million years, what fraction of the initial 14C atoms is still there? (t1/2 = 5730 yr)
A) 0
B) 1 × 10-10
C) 5.7 × 10-5
D) 1.0 × 10-3
E) 5.7 × 10-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which equation correctly represents positron decay of 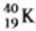 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is false?
A) Fission occurs among the heaviest isotopes, whereas fusion occurs more readily for light isotopes.
B) The mass defect (Δm) for a fission reaction is negative, whereas Δm for fusion is positive.
C) In order for fusion reactions to occur, temperatures must be in the millions of degrees.
D) The fission of Pu-239 atoms produces isotopes of many different elements.
E) Neutron-induced fission processes can occur at room temperature, rather than at millions of degrees.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclear binding energy per nucleon is defined by which of the following?
A) ![]()
![]()
B) (Nuclear binding energy) 2 × (# nucleons)
C) ![]()
![]()
D) (# nucleons) 2 × (mol of nucleons)
E) None of the answers is correct.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 127
Related Exams