A) 1.30
B) 3.00
C) 11.00
D) 12.39
E) 12.70
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these salts will form a basic solution upon dissolving in water?
A) NaCl
B) NaNO2
C) NH4NO3
D) KBr
E) AlCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is a Lewis acid but not a Brønsted acid?
A) HCN
B) CO32-
C) OH-
D) Cl-
E) Al3+
Correct Answer

verified
Correct Answer
verified
True/False
If a strong acid such as HCl is diluted sufficiently with water, the pH will be higher than 7.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below is a representation of an aqueous solution of a weak acid HA at equilibrium. (Each circle represents 1.0 ×10-3 mol of atoms, and the volume of the box is 1.0 L. Solvent water molecules are not shown for clarity.) 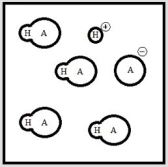 What is Ka of HA?
What is Ka of HA?
A) 1.0 ×10-6
B) 6.0 ×10-3
C) 2.5 ×10-4
D) 3.6 ×10-5
E) 1.0 ×10-9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance (CH3CH2) 2NH is considered to be
A) a weak acid.
B) a weak base.
C) a strong acid.
D) a strong base.
E) a neutral compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is [OH-] for a solution at 25°C that has [H3O+] = 2.35 × 10-3 M?
A) 4.26 × 10-5 M
B) 2.35 ×10-11 M
C) 4.26 × 10-12 M
D) 2.35 × 10-17 M
E) 2.35 ×10-3 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a Ba(OH) 2 solution is 10.00. What is the H+ ion concentration of this solution?
A) 4.0 × 10-11 M
B) 1.6 × 10-10 M
C) 1.3 × 10-5 M
D) 1.0 × 10-10 M
E) 10.00 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Equal volumes of two solutions, one containing a strong acid at pH 2 and the other containing a strong base at pH 12, are mixed. Once equilibrium is achieved, what is the final pH of the combined solution?
A) 6
B) 7
C) 10
D) 14
E) 24
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the pH of a 0.135 M aqueous solution of periodic acid (HIO4) , for which Ka = 2.3 × 10-2.
A) 1.25
B) 3.28
C) 1.17
D) 1.34
E) 1.64
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a strong acid?
A) H2CO3
B) H2SO3
C) H2SO4
D) H3PO4
E) CH3COOH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of sodium nitrite must be added to enough water to make 350.0 mL of a solution with pH = 8.40? [Ka(HNO2) = 5.6 × 10-4]
A) 68 g
B) 1.7 × 10-4 g
C) 0.039 g
D) 8.7 g
E) 24 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The acid dissociation constant Ka equals 1.26 × 10-2 for HSO4- and is 5.6 × 10-10 for NH4+. Which statement about the following equilibrium is correct? HSO4-(aq) + NH3(aq)  SO42-(aq) + NH4+(aq)
SO42-(aq) + NH4+(aq)
A) The reactants will be favored because ammonia is a stronger base than the sulfate anion.
B) The products will be favored because the hydrogen sulfate ion is a stronger acid than the ammonium ion.
C) Neither reactants nor products will be favored because all of the species are weak acids or bases.
D) The initial concentrations of the hydrogen sulfate ion and ammonia must be known before any prediction can be made.
E) This reaction is impossible to predict, since the strong acid and the weak base appear on the same side of the equation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most acidic oxides are formed from elements found in the ________ region of the periodic table.
A) upper right
B) upper left
C) center
D) lower right
E) lower left
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.20 M solution of NH4Cl? [Kb(NH3) = 1.8 × 10-5]
A) 3.74
B) 4.98
C) 6.53
D) 9.02
E) 11.28
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the weak bases below and their Kb values: C6H7O- Kb = 1.3 × 10-10 C2H5NH2 Kb = 5.6 × 10-4 C5H5N Kb = 1.7 ×10-9 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
A) C5H5NH+< C6H7OH < C2H5NH
B) C6H7OH < C5H5NH+ < C2H5NH
C) C5H5NH+< C2H5NH3+ < C6H7OH
D) C6H7OH < C2H5NH3+< C5H5NH+
E) C2H5NH3+< C5H5NH+ < C6H7OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 0.021 M NaCN solution. [Ka(HCN) = 4.9 × 10-10]
A) 1.68
B) 3.19
C) 4.69
D) 9.31
E) 10.81
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most basic oxide?
A) Bi2O3
B) SiO2
C) Cs2O
D) Na2O
E) H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the weakest acid?
A) HBrO3
B) HClO
C) HBrO2
D) HBrO
E) HClO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
After a substance acting as a strong base reacts, what remains of the base?
A) a weaker base
B) a strong conjugate acid
C) a strong conjugate base
D) a strong acid
E) a weak conjugate acid
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 137
Related Exams