A) 180 × 10²³ molecules of glucose
B) 1 kilogram of glucose dissolved in 1 liter of solution
C) 180 mL of dissolved glucose
D) 180 grams of glucose
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur is in the same column of the periodic table as oxygen, but has electronegativity similar to carbon. Compared to water molecules, molecules of H₂S will ________.
A) have greater cohesion to other molecules of H₂S
B) have a greater tendency to form hydrogen bonds with each other
C) have a higher capacity to absorb heat for the same change in temperature
D) not form hydrogen bonds with each other
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 0.01 M solution of a substance has a pH of 2. What can you conclude about this substance?
A) It is a strong acid that dissociates completely in water.
B) It is a strong base that dissociates completely in water.
C) It is a weak acid.
D) It is a weak base.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Increased atmospheric CO₂ concentrations might have what effect on seawater?
A) Seawater will become more alkaline, and carbonate concentrations will decrease.
B) There will be no change in the pH of seawater, because carbonate will turn to bicarbonate.
C) Seawater will become more acidic, and carbonate concentrations will decrease.
D) Seawater will become more acidic, and carbonate concentrations will increase.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Measurements show that the pH of a particular lake is 4.0. What is the hydrogen ion concentration of the lake?
A) 4.0 M
B) 10⁻¹⁰ M
C) 10⁻⁴ M
D) 10⁴ M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One of the buffers that contribute to pH stability in human blood is carbonic acid (H₂CO₃) . Carbonic acid is a weak acid that, when placed in an aqueous solution, dissociates into a bicarbonate ion (HCO₃⁻) and a hydrogen ion (H⁺) . (See figure.) If the pH of blood drops, one would expect ________.
A) a decrease in the concentration of H₂CO₃ and an increase in the concentration of HCO₃⁻
B) the concentration of bicarbonate ions (HCO₃⁻) to increase
C) the HCO₃⁻ to act as a base and remove excess H⁺ by the formation of H₂CO₃
D) the HCO₃⁻ to act as an acid and remove excess H⁺ by the formation of H₂CO₃
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A dietary Calorie equals 1 kilocalorie. One kilocalorie equals ________.
A) 1000 calories, or the amount of heat required to raise the temperature of 1 g of water by 1°C
B) 10,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°F
C) 1000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°C
D) 1000 calories, or the amount of heat required to raise the temperature of 100 g of water by 100°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question.
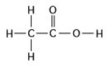 Two moles of the compound in the figure would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
Two moles of the compound in the figure would weigh how many grams? (Note: The atomic masses, in daltons, are approximately 12 for carbon, 1 for hydrogen, and 16 for oxygen.)
A) 30
B) 60
C) 90
D) 120
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that acid rain has lowered the pH of a particular lake to pH 5.0. What is the hydroxide ion concentration of this lake?
A) 1 × 10⁻⁹ mol of hydroxide ions per liter of lake water
B) 1 × 10⁻⁵ mol of hydroxide ions per liter of lake water
C) 5.0 M hydroxide ion
D) 5.0 × 10⁻⁵ mol of hydroxide ions per liter of lake water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Liquid water ________.
A) is less dense than ice
B) has a specific heat lower than that of most other substances
C) has a heat of vaporization higher than that of most other substances
D) is nonpolar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is considered to be a strong base (alkali) ?
A) HCl → H⁺ + Cl⁻
B) NH₃ + H⁺ ⇔ NH₄⁺
C) H₂CO₃ ⇔ HCO₃- + H⁺
D) NaOH → Na⁺ + OH⁻
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does ice float in liquid water?
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Stable hydrogen bonds keep water molecules of ice farther apart than water molecules of liquid water.
D) The crystalline lattice of ice causes it to be denser than liquid water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
We can be sure that a mole of table sugar and a mole of vitamin C are equal in their
A) mass.
B) volume.
C) number of atoms.
D) number of molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Melting of ice and thus reduced feeding opportunities for polar bears is occurring because of the ________.
A) increase in phytoplankton population
B) drying up of lakes and streams
C) constant breaking and reforming of hydrogen bonds in water
D) increase in CO₂ and other greenhouse gases in the atmosphere
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction at equilibrium: CO₂ + H₂O H₂CO₃. What would be the effect of adding additional H₂O?
A) It would drive the equilibrium dynamics to the right.
B) It would drive the equilibrium dynamics to the left.
C) Nothing would happen because the reactants and products are in equilibrium.
D) Reactions in both the directions will slow down.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water has many exceptional and useful properties. Which is the rarest property among compounds?
A) Water is a solvent.
B) Solid water is less dense than liquid water.
C) Water has a high heat capacity.
D) Water has surface tension.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true about buffer solutions?
A) They maintain a constant pH of 7.
B) They maintain a constant pH when acids are added to them but not when bases are added to them.
C) They fluctuate in pH when either acids or bases are added to them.
D) They maintain a relatively constant pH when either acids or bases are added to them.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A slice of pizza has 500 kcal. If we could burn the pizza and use all the heat to warm a 50-L container of cold water, what would be the approximate increase in the temperature of the water? (Note: A liter of cold water weighs about 1 kg.)
A) 50°C
B) 5°C
C) 100°C
D) 10°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thermal energy of the water in a bathtub is ________ than in a freshly brewed coffee pot because of its ________.
A) higher; greater volume
B) higher; high kinetic energy
C) lower; low temperature
D) lower; low density
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the [H₃O⁺] of the solution decreases, the [OH⁻] ________.
A) increases and thus pH increases
B) increases and thus pH decreases
C) decreases and thus the pH decreases
D) decreases and thus the pH increases
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 55
Related Exams