A) oil is nonpolar.
B) water is saturated.
C) water is nonpolar.
D) oil is hydrated.
E) oil is polar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water is a polar solvent and hexane 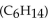 is a nonpolar solvent. Which of the following correctly describes the solubility of the solute?
is a nonpolar solvent. Which of the following correctly describes the solubility of the solute?
A) ![]() soluble in hexane
soluble in hexane
B) ![]() soluble in water
soluble in water
C) ![]() soluble in water
soluble in water
D) octane, soluble in water
E) mineral oil, soluble in water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An increase in the temperature of a solution usually
A) increases the boiling point.
B) decreases the solubility of a solid solute in the solution.
C) increases the solubility of a solid solute in the solution.
D) increases the solubility of a gas in the solution.
E) decreases the solubility of a liquid solute in the solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules can form hydrogen bonds?
A) HI
B) BH3
C) NH3
D) NaH
E) CH4
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The molarity (M) of a solution refers to
A) moles of solute/L of solvent.
B) grams of solute/100 mL of solution.
C) grams of solute/L of solution.
D) moles of solute/L of solution.
E) moles of solute/100 mL of solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds will be insoluble in water?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
0.50 mole of NaCl , a strong electrolyte, is added to 1.0 kg of water. The freezing point of the solution will be
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which one of the following compounds will be insoluble in water?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many equivalents are present in 5.0 g of 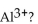
A) 0.37 Eq
B) 0.19 Eq
C) 3 Eq
D) 15 Eq
E) 0.56 Eq
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture in which one component settles is called a(n) ________.
A) nonelectrolyte
B) solution
C) colloid
D) electrolyte
E) suspension
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of 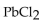 are formed when 25.0 mL of 0.654 M KCl react completely with
are formed when 25.0 mL of 0.654 M KCl react completely with 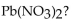
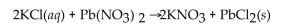
A) 4.54 g
B) 22.7 g
C) 2.27 g
D) 9.08 g
E) 1.64 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1.0 mole of NaCl, a strong electrolyte, is added to 1.0 kg of water. The freezing point of the solution will be ________ the freezing point of pure water.
A) higher than
B) lower than
C) the same as
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When KCl dissolves in water,
A) the ![]() ions are attracted to dissolved
ions are attracted to dissolved ![]() ions.
ions.
B) the ![]() ions are attracted to the partially positive hydrogen atoms of the water molecule.
ions are attracted to the partially positive hydrogen atoms of the water molecule.
C) the ![]() ions are attracted to the partially negative oxygen atoms of the water molecule.
ions are attracted to the partially negative oxygen atoms of the water molecule.
D) the ![]() ions are attracted to the partially negative oxygen atoms of the water molecule.
ions are attracted to the partially negative oxygen atoms of the water molecule.
E) the ![]() ions are attracted to
ions are attracted to ![]() ions on the \mathrm{KCl} crystal.
ions on the \mathrm{KCl} crystal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
NaCl can be classified as a ________.
A) liquid
B) strong electrolyte
C) gas
D) nonelectrolyte
E) weak electrolyte
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Henry's law, the solubility of a gas in a liquid
A) remains the same as the temperature increases.
B) decreases as the gas pressure above the liquid increases.
C) depends on the liquid polarity.
D) increases as the gas pressure above the liquid increases.
E) depends on the liquid density.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molarity of a solution that contains 3.25 moles of 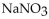 in 250 mL of solution?
in 250 mL of solution?
A) 13.0 M
B) 2.60 M
C) 3.25 M
D) 6.50 M
E) 0.0130 M
Correct Answer

verified
Correct Answer
verified
Essay
A substance that carries an electric current when dissolved in water is called a(n)________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
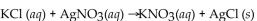 How many mL of 0.234 M KCl solution will react completely with 25.0 mL of 0.168 M
How many mL of 0.234 M KCl solution will react completely with 25.0 mL of 0.168 M 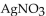 solution?
solution?
A) 35.6 mL
B) 17.9 mL
C) 34.8 mL
D) 25.0 mL
E) 22.5 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When solutions of NaCl and 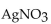 are mixed,
are mixed,
A) a precipitate of AgCl forms.
B) precipitates of ![]() and AgCl form.
and AgCl form.
C) a precipitate of ![]() forms.
forms.
D) no precipitate forms.
E) a precipitate of ![]() forms.
forms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When some of the sugar added to iced tea remains undissolved at the bottom of the glass, the solution is
A) saturated.
B) polar.
C) dilute.
D) nonpolar.
E) unsaturated.
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 84
Related Exams