A) sugar molecules from the concentrated to the dilute solution.
B) sugar molecules from the dilute to the concentrated solution.
C) water molecules from the concentrated to the dilute solution.
D) water molecules from the dilute to the concentrated solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iodine, I2, is very slightly soluble in water, a polar solvent, but quite soluble in toluene, a nonpolar solvent. What can be inferred about the nature of the I2 molecule?
A) It is ionic.
B) It is polar.
C) It is nonpolar.
D) Nothing can be inferred.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ability to see the scattering of light when passed through a colloid is known as
A) the dispersing effect.
B) a scattering ratio.
C) an emulsifying agent.
D) the Tyndall effect.
Correct Answer

verified
D
Correct Answer
verified
True/False
There is a 12 M aqueous HCl solution in the stock room, but a 6 M solution is required for an experiment. Doubling the volume of a 12 M sample with water will produce a 6 M solution.
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
Which of the following is not considered a colligative property?
A) vapor pressure
B) boiling point
C) conductivity
D) freezing point
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements relating to a solution is not correct?
A) A solution may contain more than one solute.
B) A solution may contain only one solute.
C) Water is always the solvent in a solution.
D) More than one correct response given.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A solution is prepared at 75 °C by dissolving 50.0 g of A in 100 g of water. When this solution is cooled to 15 °C what happens based on the following solubility plot for A. 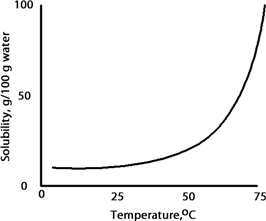
A) Solid A crystallizes and the solution is unsaturated.
B) Solid A dissolves and the solution is supersaturated.
C) Solid A crystallizes and the solution is saturated.
D) Solid A dissolves and the solution is unsaturated.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of ethyl alcohol is contained in 35 mL (1 oz.) of 86 proof liquor, which is 43% (v\v) alcohol?
A) 30 mL
B) 43 mL
C) 15 mL
D) 37 mL
Correct Answer

verified
Correct Answer
verified
True/False
Ionic compounds are generally insoluble in non-polar solvents.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Drinking water can be purified by which of the following methods?
A) reverse osmosis
B) distillation
C) ion exchange
D) all of the choices
Correct Answer

verified
Correct Answer
verified
True/False
Solvents and hydrated ions can usually pass though dialyzing membranes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared by adding 25.0 mL of 1.30 M AlCl3 solution to a flask, and then adding enough water to give a final volume of 200.0 mL. What is the molarity of the solution?
A) 0.260
B) 0.163
C) 6.50
D) 1.24
Correct Answer

verified
Correct Answer
verified
True/False
The more soluble a substance is, the faster it will dissolve.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When sodium acetate dissolves in water, the following reaction occurs. 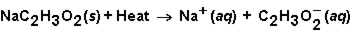 This solution could be used
This solution could be used
A) in a hot pack.
B) in a cold pack.
C) either a hot pack or cold pack.
D) neither a hot pack or cold pack.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution?
A) 0.100
B) 1.00
C) 0.0250
D) 0.400
Correct Answer

verified
Correct Answer
verified
True/False
Dialysis and osmosis are used for the same purposes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is made by dissolving 15.0 mL of oil in enough gasoline to give 50.0 mL of solution. What is the % (v\v) of oil in the solution?
A) 30.0
B) 23.1
C) 42.9
D) 3.33
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compared to pure water, a salt water solution will have a
A) lower vapor pressure, freezing point and boiling point.
B) higher vapor pressure, freezing point and boiling point.
C) lower vapor pressure and freezing point and a higher boiling point.
D) higher freezing point and a lower vapor pressure and boiling point.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is most soluble in a polar solvent?
A) silver nitrate
B) silver chloride
C) silver carbonate
D) all are of equal solubility
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The vapor pressure of a pure solvent is
A) higher than that of a solution.
B) impossible to determine.
C) the same as that of a solution.
D) lower than that of a solution.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 98
Related Exams