A) B
B) C
C) O
D) F
E) Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What quantum number(s) is(are) required to completely describe a subshell , a set of orbitals with the same energy and shape? I. Principal quantum number II. Angular Momentum quantum number III. Magnetic quantum number
A) I only
B) I and II
C) I and III
D) II and III
E) I, II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element with a ground state electron configuration of 1s22s22p4 is
A) B
B) C
C) O
D) F
E) Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can occupy a single 3px atomic orbital ?
A) zero
B) one
C) two
D) three
E) six
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three sets of quantum numbers are listed below. Pick the best answer.
I. n = 2, 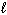 = 2,
= 2, 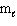 = 2, m s = 1 / 2
II. n = 4,
= 2, m s = 1 / 2
II. n = 4, 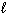 = 0,
= 0, 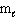 = 0, m s = 1 / 2
III. n = 3,
= 0, m s = 1 / 2
III. n = 3, 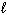 = 2,
= 2, 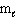 = 3, m s = 1 / 2
= 3, m s = 1 / 2
A) I and II are allowed sets, III is not
B) II and III are allowed sets, I is not
C) all 3 are allowed sets
D) only II is an allowed set
E) all 3 are not allowed sets
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground state electron configuration of oxygen?
A) 1s22s22p4
B) 1s22s22p2
C) 1s22s22p3
D) 1s22s23s4
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many possible orientations are allowed when 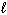 = 2?
(d-type sublevel; How many possible
= 2?
(d-type sublevel; How many possible 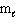 values are possible?)
values are possible?)
A) 1
B) 2
C) 3
D) 5
E) 7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If waves are hitting the beach every 2.0 seconds and are 12 m apart, what is the velocity of the waves?
A) 6.0 m/s
B) 24 m/s
C) 18 m/s
D) 2.0 m/s
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain blue light has a wavelength oF453 nm ( l = 453 nm) . What is the energy (in Joules) of one photon of this blue light with this wavelength?
A) 9.97×10 - 49 J
B) 2.99×10 - 40 J
C) 1.46×10 - 27 J
D) 4.37×10 - 19 J
E) 6.86×1026 J
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the maximum number of electrons that can occupy a 5f subshell?
A) 2
B) 5
C) 6
D) 10
E) 14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct ground state electronic configuration of Lead (Z = 82) ?
A) 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p2
B) [Xe]6s26d106p2
C) [Xe]6s26f146d106p2
D) [Xe]6s24f145d106p2
E) [Xe]6s26p2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the frequency of light whose wavelength is 5.0×10 - 6 meters.
A) 6.0×1013 s - 1
B) 2.0×105 s - 1
C) 3.0×108 s - 1
D) 4.0×10 - 20 s - 1
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the frequency of light emitted when an electron in a hydrogen atom goes from the n = 5 shell to the n = 2 shell.
A) 6.9×1014 s - 1
B) 8.2×1013 s - 1
C) 7.8×1014 s - 1
D) 1.2×1014 s - 1
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The frequency of a photon of electromagnetic radiation with an energy of 7.3×10 - 18 J is
A) 2.7×10 - 8 s - 1
B) 9.9×1019 s - 1
C) 2.0×1016 s - 1
D) 1.1×1016 s - 1
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One type of sunburn occurs on exposure to UV light of wavelength equal to 325 nm. What is the frequency given in sec - 1 for this wavelength?
A) 1.08×10 - 15 sec - 1
B) 9.23×10 - 4 sec - 1
C) 9.75×101 sec - 1
D) 9.23×105 sec - 1
E) 9.23×1014 sec - 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What quantum number(s) provide(s) information with respect to the size of an atomic orbital?
A) Principal quantum number, "n"
B) Angular Momentum quantum number, "l"
C) Magnetic quantum number, "ml"
D) Electron spin quantum number, "ms"
E) Principle and Angular Momentum quantum numbers
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
How many unpaired electrons are present in a neutral, ground state nitrogen atom?
A) 0
B) 1
C) 3
D) 5
E) 7
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the correct electronic configuration for Nickel (Z = 28) ?
A) [Ar]3d10
B) 1s22s22p63s23p63d84s2
C) 1s22s22p63s23p63d10
D) 1s22s22p63s23p64s23d8
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the order for filling the following subshells with electrons? 4d, 4f, 5s, 5p, 6s
A) 4d, 4f, 5s, 5p, 6s
B) 5s, 6s, 5p, 4d, 4f
C) 5s, 4d, 5p, 6s, 4f
D) 4d, 4f, 5p, 5s, 6s
E) 6s, 5p, 5s, 4f, 4d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the restrictions on the three quantum numbers that define an orbital, which set of three quantum numbers listed below is forbidden ?
A) n = 3, ![]() = 3,
= 3, ![]() = 3
= 3
B) n = 3, ![]() = 2,
= 2, ![]() = - 2
= - 2
C) n = 3, ![]() = 2,
= 2, ![]() = +2
= +2
D) n = 3, ![]() = 1,
= 1, ![]() = +1
= +1
E) n = 3, ![]() = 0,
= 0, ![]() = 0
= 0
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 86
Related Exams