A) IF3
B) NO2
C) BF3
D) SeCl4
E) PF6 - 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the polyatomic ion, (PO3) 3 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the formula of an ionic compound formed from barium and sulfur.
A) BaS
B) Ba2S
C) Ba S2
D) Ba3S
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons (both lone and bond pairs) are used in the Lewis structure of C2O42 - ?
A) 30
B) 34
C) 32
D) 46
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons (both lone and bond pairs) are used in the Lewis diagram of CH2NH?
A) 16
B) 12
C) 14
D) 10
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Possible resonance forms for ClO3 - are shown below. Pick the best answer (formal charges are circled) .
I. 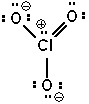 II.
II. 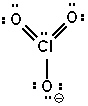 III.
III. 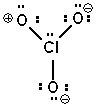
A) I and II are correct, III is not
B) only III is correct
C) II and III are correct, I is not
D) all are incorrect
E) all are correct
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below obeys the octet rule for every atom in the structure of Br2 and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the average bond energies listed below, estimate D H for the following reaction: 3 H2 + N2→2 NH3 D H rxn = ?? Average Bond Energies: H - H436 kJ/mol N º N 946 kJ/mol N - N 163 kJ/mol N - H 389 kJ/mol
A) - 80 kJ
B) 1087 kJ
C) - 120 kJ
D) 220 kJ
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons (both lone and bond pairs) are shown in the correct Lewis structure of HClO?
A) 14
B) 12
C) 16
D) 20
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a valid resonance form for N2O?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) All of these are valid resonance forms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is(are) the driving force(s) for formation of an ionic compound from its elements?
A) A large amount of heat is released when two oppositely charged ions come together to form a crystal lattice.
B) Both elements in the compound attain a stable Noble gas electronic configuration through a complete transfer of electrons from the metal to the nonmetal.
C) Both elements in the compound attain a stable Noble gas electronic configuration through sharing of electrons.
D) Both a and b
E) Both a and c
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below for hydrazine, N2H4, obeys the octet rule and contains the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom from the list below normally forms two covalent bonds ?
A) N
B) F
C) C
D) Br
E) O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the polyatomic ion, CN1 - . Which Lewis structure below obeys the octet rule for both atoms in the structure and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical property of hydrogen, H₂ , is that it reacts with oxygen, O₂, to produce water, H₂ O (a molecular compound) , as shown below. How does each element in the compound H₂ O attain a Noble gas electronic configuration?
2 H₂ (g) + O₂(g) 2 H₂ O (  )
)
A) An oxygen atom loses two electrons and each hydrogen atom accepts an electron forming O2+ and two H - ions.
B) Each hydrogen atom loses an electron and the oxygen atom loses six electrons.
C) Each hydrogen atom loses an electron and the oxygen atom accepts the two electrons forming 2 H+ ions and one O2 - ion.
D) Each hydrogen atom gains seven electrons and the oxygen atom loses six electrons.
E) Each hydrogen atom and the oxygen atom share two electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure below for CO2 obeys the octet rule for every atom in the structure and uses the proper number of valence electrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons (both lone and bond pairs) are shown in the Lewis diagram of CO32 -?
A) 20
B) 22
C) 24
D) 28
E) 30
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Pick the correct Lewis structure shown below.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) all 4 are incorrect
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the three statements below, pick the best answer.
I. The Lewis symbol of boron is 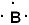 II. KCl should have a greater lattice energy than BeO.
III. The Lewis symbol Mg is correct for a magnesium atom.
II. KCl should have a greater lattice energy than BeO.
III. The Lewis symbol Mg is correct for a magnesium atom.
A) all three are true
B) I and II are true, III is false
C) I and III are true, II is false
D) II and III are true, I is false
E) only III is true
Correct Answer

verified
Correct Answer
verified
Showing 101 - 119 of 119
Related Exams