A) D G ° rxn = 7.31×104 kJ/mol
B) D G ° rxn = - 41.0 kJ/mol
C) D G ° rxn = - 89.4 kJ/mol
D) D G ° rxn = - 187.5 kJ/mol
E) D G ° rxn = - 203.6 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The entropy change when one mole of acetone [(CH3) 2CO] vaporizes at its boiling point (56 ° C) is D S vap = 97.16 J/mol × K. How much heat is absorbed when 58.0 grams of acetone vaporizes at 56 ° C?
A) 0.29 kJ
B) 5.44 kJ
C) 97.16 kJ
D) 234 J
E) 32.0 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given D G rxn = - 120.4 kJ/mol for the following reaction, what is the value of its equilibrium constant, K , at 298 K?
H₂ (g) + O₂(g)  H₂ O₂ (
H₂ O₂ (  )
)
A) K = 7.7×10 - 22
B) K = 1.1
C) K = 49
D) K = 2.9×101
E) K = 1.3×1021
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units express(es) a quantity of energy? I. joule II. L × atm III. kcal
A) I and III, but not II
B) I and II, but not III
C) I, II, and III
D) I only
E) none of them
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction PCl3 (g) + Cl2 (g) →PCl5 (g) is exothermic. The reaction is:
A) spontaneous at all temperatures.
B) nonspontaneous at all temperatures.
C) spontaneous at low temperatures.
D) spontaneous at high temperatures.
E) spontaneous if D G rxn is positive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 17-5 Consider the reaction of Barium oxide with carbon dioxide to form Barium Carbonate as shown below to answer the following question(s) . BaO (s) + CO2 (g) →BaCO3 (s) D S ° rxn = - 174 J/mol × K and D H ° rxn = - 277 kJ/mol -Refer to Exhibit 17-5. Which statement below is true?
A) This reaction is spontaneous at all temperatures.
B) This reaction is non-spontaneous at all temperatures.
C) This reaction is spontaneous at relatively lower temperatures and non-spontaneous at higher temperatures.
D) This reaction is spontaneous at relatively higher temperatures and non-spontaneous at lower temperatures.
E) There is not enough information to make an assessment.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction and its standard molar Gibbs Free-Energy of reaction:
2 Fe3+ (aq) + Hg 2 2+ (aq)  2 Fe2+ (aq) + 2 Hg 2+ (aq) D G rxn = 2.87 kJ/mol What is the molar Gibbs Free-Energy of reaction at 500 K under non-standard conditions when Fe3+ = 0.25 M, Hg 2 2+ = 0.33 M, Fe2+ = 0.15 M and Hg 2+ = 0.32 M?
2 Fe2+ (aq) + 2 Hg 2+ (aq) D G rxn = 2.87 kJ/mol What is the molar Gibbs Free-Energy of reaction at 500 K under non-standard conditions when Fe3+ = 0.25 M, Hg 2 2+ = 0.33 M, Fe2+ = 0.15 M and Hg 2+ = 0.32 M?
A) D G rxn = - 6.24 kJ/mol
B) D G rxn = 0.619 kJ/mol
C) D G rxn = 2.78 kJ/mol
D) D G rxn = 9.11 kJ/mol
E) D G rxn = 12.0 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a given chemical reaction, the value of D H is negative and the value of D S is positive. The value of D G for this reaction indicates that the reaction is:
A) always nonspontaneous.
B) spontaneous only at high temperatures.
C) spontaneous only at low temperatures.
D) spontaneous at all temperatures.
E) nonspontaneous only at high temperatures.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of a 3.06 g sample of formic acid (H CO₂ H(  ) , molar mass = 46.03 g/mol) in a bomb calorimeter with a heat capacity of 5.423 J/ C causes the temperature to rise from 23.27 C to 26.41 C. What is D E for the reaction?
2 H CO₂ H (
) , molar mass = 46.03 g/mol) in a bomb calorimeter with a heat capacity of 5.423 J/ C causes the temperature to rise from 23.27 C to 26.41 C. What is D E for the reaction?
2 H CO₂ H (  ) + O₂(g) 2 CO₂ (g) + 2 H₂ O (
) + O₂(g) 2 CO₂ (g) + 2 H₂ O (  )
)
A) - 5.56 kJ
B) - 17.0 kJ
C) - 256 kJ
D) - 512 kJ
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following processes will D S system be the most positive?
A) H2 (g) + Cl2 (g) → 2 HCl (g)
B) 2 H₂ (g) + O₂(g) 2 H₂ O ( ![]() )
)
C) CaCO3 (s) → CaO (s) + CO2 (g)
D) Ag+ (aq) + Cl - (aq) → AgCl (s)
E) KClO4 (s) → KCl (s) + 2 O2 (g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes is(are) considered spontaneous ?
I. Melting ice cubes at 5 C and 1 atm.
II. Rust, Fe2 O3 , is converted to iron, Fe, and oxygen, O₂.
III. Forming additional NH₃from the following reaction that has previously reached a state of dynamic equilibrium:
N₂(g) + 3 H₂ (g) 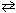 2 NH₃(g)
2 NH₃(g)
A) I only
B) II only
C) III only
D) I and II
E) All of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If D H is positive and D S is negative for a particular reaction:
A) the reaction proceeds forward at all temperatures.
B) the reaction proceeds forward at low temperatures.
C) the reaction proceeds forward at high temperatures.
D) the reaction doesn't proceed forward at any temperature.
E) none of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equilibrium constant, K eq , for the following reaction at 25 C, given that D G rxn = - 16.8 kJ/mol?
H₂ (g) + I2 (g) 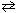 2 HI (g)
2 HI (g)
A) K eq = 1.01
B) K eq = 6.78
C) K eq = 884
D) K eq = 6.08×106
E) K eq = 1.32×1035
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of a 0.440 g sample of ethanol (C₂ H5 OH, molar mass = 46.07 g/mol) in a bomb calorimeter with a heat capacity of 5.278 kJ/ C causes the temperature to rise from 23.98 C to 26.45 C. What is D E for the reaction?
C₂ H5 OH (  ) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O (
) + 3 O₂(g) 2 CO₂ (g) + 3 H₂ O (  )
)
A) - 13.0 kJ
B) - 1.36×103 kJ
C) - 29.6 kJ
D) +13.0 kJ
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes results in a negative entropy change?
A) HCN(g, V = 10 L, P = 4 atm) ![]() HCN (g, V = 20 L, P = 2 atm)
HCN (g, V = 20 L, P = 2 atm)
B) 2 HI (g) ![]() H₂ (g) + I2 (s)
H₂ (g) + I2 (s)
C) NaI (s) ![]() NaI (
NaI ( ![]() )
)
D) CaCO3(s) ![]() CaO (s) + CO₂ (g)
CaO (s) + CO₂ (g)
E) Bi ( ![]() ) + Sn (
) + Sn ( ![]() ) + Pb (
) + Pb ( ![]() )
) ![]() Soft Solder (
Soft Solder ( ![]() )
)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions would be expected to have a positive entropy change , D S > 0? I. 3 O2 (g) →2 O3 (g) II. CaCO3 (s) →CaO (s) + CO2 (g) III. C3H8 (g) + 5 O2 (g) →3 CO2 (g) + 4 H2O (g)
A) I only
B) II only
C) III only
D) I and II
E) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions would be expected to provide a negative change in entropy , D S I. 2 K (s) + F2 (g) →2 KF (s) II. 2 NO2 (g) →N2O4 (g) III. NaClO3 (s) →Na+ (aq) + ClO31 - (aq)
A) I only
B) II only
C) III only
D) I and II
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 17-4 Consider the reaction of nitrogen monoxide and oxygen gas to form nitrogen dioxide as shown below to answer the following question(s) . NO (g) + O2 (g) →2 NO2 (g) -Refer to Exhibit 17-4. Which statement below is correct about the reaction above?
A) This reaction is spontaneous at all temperatures.
B) This reaction is non-spontaneous at all temperatures.
C) This reaction is spontaneous at relatively lower temperatures and non-spontaneous at higher temperatures.
D) This reaction is spontaneous at relatively higher temperatures and non-spontaneous at lower temperatures.
E) There is not enough information to make an assessment.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given K p = 0.100 at 25 C for N₂O4 (g) 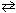 2 NO₂ (g) , what is the value of D G reaction at equilibrium?
2 NO₂ (g) , what is the value of D G reaction at equilibrium?
A) 0.479 kJ/mol
B) - 5.70 kJ/mol
C) 0.00 kJ/mol
D) positive since the reaction is spontaneous
E) negative since the reaction is nonspontaneous
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which thermodynamic statement listed below would confirm that a chemical reaction is spontaneous as written?
A) D G < 0
B) D G > 0
C) D S < 0
D) D H > 0
E) D H > 0 and D S < 0
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 106
Related Exams