A) H+ + OH- → H2O
B) Li+ + NO3- → LiNO3
C) LiOH + HNO3 → LiNO3 + H2O
D) LiOH → Li+ + OH-
E) HNO3 → H+ + NO3-
F) Li+ + OH- + H+ + NO3- → LiNO3+ H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is the most alkaline?
A) coffee (pH 5.12)
B) urine (pH 7.24)
C) dishwasher detergent (pH 2.38)
D) drain cleaner (pH 10.24)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
In an aqueous solution. the [OH-] is 2.0 × 10-3 M. What is the [H3O+] of this solution?
A) 5.0 × 10-12 M
B) 2.0 × 1011 M
C) 2.0 × 10-3 M
D) 5.0 × 1012 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the conjugate base of H3PO4?
A) H2PO4-
B) HPO42-
C) PO43-
D) OH-
E) both H2PO4- and HPO42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is characteristic of a buffer?
A) The pH will go down significantly when H3O+ is added to the buffer.
B) The pH will go down very slightly when H3O+ is added to a buffer.
C) The pH will go up significantly when H3O+ is added to the buffer.
D) The pH will go up very slightly when H3O+ is added to the buffer.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is a polyprotic acid?
A) HCl
B) HNO3
C) H2SO4
D) H3PO4
E) both c and d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a cause of respiratory acidosis?
A) a poor diet
B) hyperventilation
C) hypoventilation
D) intense exercise
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of a buffer prepared with equal concentrations of an acid and its conjugate base?
A) pH = 7
B) pH = pKa
C) The pH depends on the concentration of the buffer.
D) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a solution of HF (a weak acid) is added to a solution containing HPO42- (here, a base) , the equation for reaction that occurs is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
The following solutions are ranked from the most acidic to the most basic. Most acidic urine (pH 5.89) intestine contents (pH 8.06) blood (pH 7.30) Most basic
Correct Answer

verified
Correct Answer
verified
Essay
Write the equation for the ionization of the weak acid, formic acid, HCHO2, in water.
Correct Answer

verified
HCHO2(aq) +...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
All of the following solutions are 0.025 M, which of the solutions contains the highest concentration of OH-?
A) acetic acid (pH 3.17)
B) ascorbic acid (pH 6.70)
C) phenol (pH 5.73)
D) iodic acid (pH 1.60)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the image which depicts the reaction between CH3COOH and H2O. Reactants: Products: 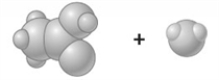
 If the composition of the equilibrium mixture is as represented below
If the composition of the equilibrium mixture is as represented below 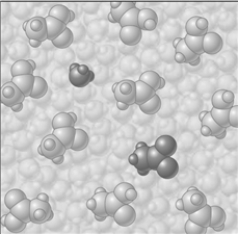 CH3COOH is classified as
CH3COOH is classified as
A) strong electrolyte
B) weak electrolyte
C) nonelectrolyte
Correct Answer

verified
Correct Answer
verified
Essay
If you dissolve 0.10 mol of a generic acid HA in 1.0 L of solution, the pH is 3.5. Is HA a strong or weak acid? Explain your answer.
Correct Answer

verified
HA is a weak acid that is only partially...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
The following represents the structure of a hydronium ion. 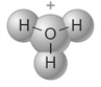
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a solution of KOH is added to a solution of HCO2H (formic acid) , which of the following would be shown in the molecular equation as a product of the reaction?
A) H2O
B) KH
C) K-
D) KCO2H
E) both H2O and KCO2H
F) both H2O and KH
Correct Answer

verified
Correct Answer
verified
Short Answer
NaOH and H2SO4 are mixed and allowed to react. How many grams of NaOH are required to neutralize 17.25 g of H2SO4?
Correct Answer

verified
Correct Answer
verified
True/False
Consider the following reaction. CH3OH(l) + CH3OH(l) 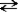 CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sodium hydroxide solution has a pH of 12.40, what is the OH- concentration in this solution?
A) 4.0 × 101 M
B) 4.0 × 10-13 M
C) 2.5 × 10- 2 M
D) 2.5 × 1012 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When fumaric acid, H2C4H2O4 reacts with NaOH, the second reaction that occurs is
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 85
Related Exams