A) 14
B) 16
C) 18
D) 20
E) 26
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw a Lewis structure for H3C-NH2. Based on this Lewis structure, the calculated value for the formal charge on the nitrogen atom is Hint: Do not forget about double or triple bonds potentially being in the Lewis structure.
A) 2-.
B) 3+.
C) 3-.
D) 2+.
E) 0.
Correct Answer

verified
Correct Answer
verified
True/False
There are five lone pairs of electrons in the acetate ion, CH3COO-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The metaphosphate ion, PO3-, is the structural analog of the NO3- ion with respect to the arrangement of the atoms in the ion. After drawing the "best"Lewis structure for the metaphosphate ion based on formal charge considerations, what is the number of resonance structures for this "best"Lewis structure? Hint: Use formal charge to help determine how many realistic resonance structures there are for the metaphosphate ion.
A) 1 (no resonance structures)
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the fluoride ion reacts with a BF3 molecule (a molecule in which there are no multiple bonds) , an ion is formed in which the boron atom is the central atom. The bond between the boron trifluoride and the fluoride ion is
A) an ionic bond.
B) a regular covalent bond, where both species contribute 1 electron to the bond.
C) a coordinate covalent bond.
D) a resonance hybrid bond.
E) a bond where two atoms share one electron instead of two.
Correct Answer

verified
Correct Answer
verified
Short Answer
The total number of valence electrons used in the C4H10 molecule is ________ so there is a total of ________ covalent bonds in this hydrocarbon molecule ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the H°f data given, which compound is the most stable?
A) CO2(g) , -393.5 kJ/mol
B) NO2(g) , +33.85 kJ/mol
C) NO(g) , +90.4 kJ/mol
D) CO(g) , -110.5 kJ/mol
E) NH3(g) , -46.4 kJ/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the electron configuration of the metal ion in Cr2(SO4)3?
Correct Answer

verified
Correct Answer
verified
Short Answer
The total number of non-bonding electrons in the NF3 molecule is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Of the following solids, NaI, NaF, MgO, MgCl2, and KF, the solid with the highest melting point is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For two ions with charges q1 and q2 separated by distance r, the potential energy canbe calculated from Coulomb's law: E = 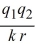 Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and
Calculate the energy released when 1 mole NaCl is formed if the constant k = 1.11 × 10-10 C2/J·m, the charge Na+ = +1 e, and the charge on Cl- is -1 e, e = 1.602 × 10-19 C, and 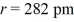
A) +308 kJ/mol
B) +8.20 kJ/mol
C) -297 kJ/mol
D) -494 kJ/mol
E) -371 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ion of uranium has a noble gas electron configuration?
A) U2+
B) U3+
C) U4+
D) U6+
E) U2-
Correct Answer

verified
Correct Answer
verified
True/False
When the Lewis structure for the nitrite ion is drawn, based on formal charge considerations, there are four bonds in its structure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the nitrogen monoxide molecule, the dipole moment is 0.16 D and the bond length is 115 pm. Determine the sign and magnitude of the charge on the oxygen atom. Hint: Watch your units carefully with this problem.
A) -1.3 e-
B) -0.029 e-
C) -0.097 e-
D) -0.72 e-
E) +1.3 e-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ion has a noble gas electron configuration?
A) Fe3+
B) Sn2+
C) Ni2+
D) Ti4+
E) Cr3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following in terms of decreasing electronegativity values.P, O, As, S Hint: Remember to refer to your periodic table for trends.
A) P > O > As > S
B) As > P > S > O
C) S > O > P > As
D) As > S > P > O
E) O > S > P > As
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for HNO2. If the valence shells are filled to the usual limit (a maximum of 8) , how many nonbonding valence electrons are in the molecule?
A) 0
B) 2
C) 6
D) 10
E) 14
Correct Answer

verified
Correct Answer
verified
Short Answer
The Lewis symbol for the oxygen atom shows ________ valence shell electrons. The number of covalent bonds that oxygen normally forms to complete its valence shell and obey the octet rule is ________.
Correct Answer

verified
Correct Answer
verified
True/False
Based on the skeleton structure, N-N-O, the magnitude of the sum of the partial charges for N2O has to be 4. Hint: Write out the full Lewis structure and remember your double and triple bonds.
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the data to calculate the lattice energy of lithium chloride. 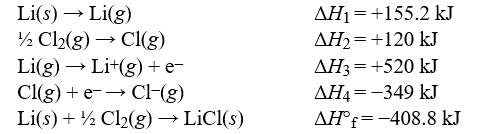
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 167
Related Exams