A) 1.08 × 103 m/s
B) 3.96 × 104 m/s
C) 4.48 × 105 m/s
D) 4.05 × 106 m/s
E) 2.77 × 102 m/s
Correct Answer

verified
Correct Answer
verified
Short Answer
When an atom absorbs energy it moves from its ground state to its ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The notation for the subshell with n = 5 and l = 3 is
A) 5d subshell.
B) 5p subshell.
C) 5f subshell.
D) 5g subshell.
E) 5s subshell.
Correct Answer

verified
Correct Answer
verified
Short Answer
Which of the following choices is the correct electron configuration for a vanadium atom? 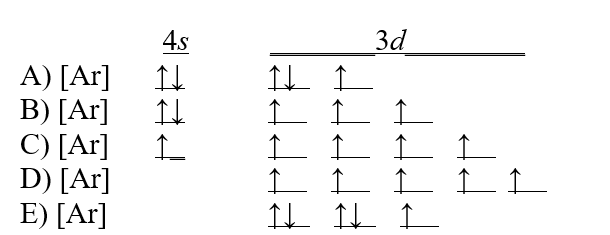
Correct Answer

verified
Correct Answer
verified
Short Answer
The very narrow band of wavelengths ranging from about 400 to 700 nm is called the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom has the smallest first ionization energy?
A) Rb
B) Na
C) Al
D) Ne
E) O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about a hydrogen atom is false?
A) Atoms undergo the same specific energy changes.
B) When an excited atom loses energy, only a specific amount can be lost.
C) When an atom is supplied with energy, an electron drops from a higher energy level to a lower energy level.
D) When an electron drops back to a lower energy level, energy equal to the difference between the two levels is released and emitted as a photon.
E) The statements above are all true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the aufbau principle and other applicable guiding principles, what electron configuration would one reasonably expect to find for technetium (Z = 43) ?
A) [Kr] 4s2 3d5
B) [Kr] 4s2 4d5
C) [Kr] 4d7
D) [Kr] 5s2 4d5
E) [Kr] 5s2 5d5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 2 and n2 = 4.Hint: Use the Rydberg equation to solve.
A) 207 nm
B) 365 nm
C) 486 nm
D) 274 nm
E) 131 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is true?
A) The line spectra of atoms consist of a series of white lines superimposed on a colorful background.
B) The line spectra of atoms consist of a series of white lines superimposed on a dark background.
C) The line spectra of atoms consist of a series of colorful lines superimposed on a dark background.
D) The line spectra of atoms consist of a series of dark lines superimposed on a white background.
E) The line spectra of atoms consist of a series of dark lines superimposed on a colorful background.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are expected to be paramagnetic in their ground state electron configurations: S, Ne, Cd, Si, Cl? Hint: Look for unpaired electrons.
A) S, Ne, and Cl
B) Cd, Ne, and Cl
C) Cd, Ne, and Si
D) S, Si, and Cl
E) All are paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the frequency of visible light having a wavelength of 464.1 nm.
A) 139.1 s-1
B) 1.548 × 10-6 s-1
C) 1.548 × 10-15 s-1
D) 6.464 × 1014 s-1
E) 6.464 × 105 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The values of n for the valence shells of Sb, Ca, I, and Po are, respectively:
A) 3, 4, 5, 6
B) 5, 5, 6, 6
C) 3, 4, 3, 6
D) 5, 6, 5, 4
E) 5, 4, 5, 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A correct description for the electron configuration of a vanadium atom is
A) [Ar] 4s1 3d4, paramagnetic.
B) [Ar] 4s2 3d3, paramagnetic.
C) [Ar] 4s3 3d2, paramagnetic.
D) [Ar] 4d5, paramagnetic.
E) [Ar] 3s2 3d3, paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the frequency of radiation which has an energy of 216.1 kJ per mole of photons?Hint: Pay careful attention to your units. 1 kJ = 1000 J.
A) 615.9 × 1014 s-1
B) 1.624 × 1014 s-1
C) 1.058 × 10-10 s-1
D) 5.416 × 1014 s-1
E) 3.588 × 10-19 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom has the smallest radius?
A) Rb
B) Na
C) Al
D) Ne
E) O
Correct Answer

verified
Correct Answer
verified
True/False
Atomic hydrogen has a single electron, and therefore produces the simplest emission spectrum with the fewest lines.
Correct Answer

verified
Correct Answer
verified
True/False
It is possible for three of the four quantum numbers to be the same for a pair of electrons.
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 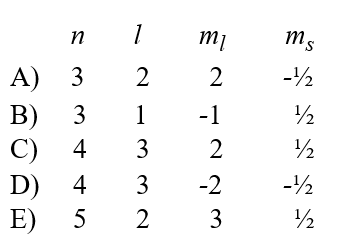
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy difference between the two energy levels that are responsible for the line that has a wavelength of 285.2 nm in an emission spectrum of an atom.
A) 4.017 × 10-21 J
B) 3.043 × 10-18 J
C) 5.192 × 10-15 J
D) 7.254 × 10-18 J
E) 6.965 × 10-19 J
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 219
Related Exams