A) 0.256 moles
B) 0.364 moles
C) 2.75 moles
D) 3.90 moles
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the % mass of sodium in sodium sulfate, Na2SO4.
A) 16%
B) 23%
C) 32%
D) 54%
E) 67%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Apatite is a mineral that is found in tooth enamel. When fluoride toothpastes are used, this mineral is converted to fluoroapatite, which is much harder and therefore more resistant to decay. What is the percent by weight of fluorine in fluoroapatite, Ca5(PO4) 3F?
A) between 0 and 0.1%
B) between 0.1% and 1%
C) between 1% and 3%
D) between 3% and 5%
E) more than 5%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the number of moles in 12.5 g of carbon tetrachloride, CCl4.
A) 8.13 x 10-2 mol
B) 12.3 mol
C) 1.92 x103 mol
D) 7.53 x 1024 mol
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of CO2 gas are produced from the combustion of 10.0 grams of isooctane? 2 C8H18 + 25 O2 16 CO2 + 18 H2O
A) 1.58 g
B) 3.85 g
C) 14.2 g
D) 30.8 g
E) 35.0 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.50 g sample of a bronze gong was cut out and dissolved in sulfuric acid. The copper sulfate produced was mixed with KI to form CuI and the triiodide ion, I3-. The I3- ion was then titrated with thiosulfate, S2O32-. Cu(s) + 2 H2SO4(aq) CuSO4(aq) + SO2(g) + 2 H2O(l) 2 CuSO4(aq) + 5 I-(aq) 2 CuI(s) + I3-(aq) + 2 SO42-(aq) I3-(aq) + 2 S2O32-(aq) → 3 I-(aq) + S4O62-(aq) If it took 31.5 mL of 1.00 M thiosulfate for this titration, how many moles of copper were present in the original 2.50 g sample?
A) 0.0105
B) 0.0158
C) 0.0315
D) 0.0630
E) 0.0945
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
How many grams of NaNO3 are required to prepare 2.00 L of a 0.250 M NaNO3 solution?
A) 0.500 g
B) 84.9 g
C) 1.70 x 102 g
D) 42.5 g
E) 6.80 x 102 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If adding 22.7 mL of 0.6 M HCl to 1.00 L of a solution of AgNO3 precipitated 1.95 g of AgCl, what was the concentration of the original AgNO3 solution? AgNO3(aq) + HCl(aq) AgCl(s) + HNO3(aq)
A) 1.36 x 10-5 M
B) 1.36 x 10-2 M
C) 0.027 M
D) 0.68 M
E) 13.6 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ethyl alcohol, C2H5OH, is also known as drinking alcohol. It can be produced by the fermentation of glucose by the following reaction. C6H12O6(aq) 2 C2H5OH(aq) + 2 CO2(g) How many of ethanol can be produced from of glucose?
A) 100 moles
B) 200 moles
C) 0.556 moles
D) 1.11 moles
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One way of determining blood alcohol levels is by titrating a sample of blood according to the following net ionic equation. C2H5OH(aq) + 2 Cr2O72-(aq) + 16 H+(aq) 2 CO2(g) + 4 Cr3+(aq) + 11 H2O(l) If 8.76 mL of 0.04988 M Cr2O72- is required for the titration of a 10.00 mL sample of blood, what was the molarity of the alcohol present in the blood sample?
A) less than 0.01 M
B) between 0.01 and 0.03 M
C) between 0.03 and 0.06 M
D) between 0.06 and 0.09 M
E) more than 0.1 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much HgS forms when 113 mL of a 0.75 M CaS solution is mixed with 52 mL of a 1.21 M Hg(NO3) 2 solution?
A) 6.30 g
B) 8.48 g
C) 14.6 g
D) 19.7 g
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In March of 2007 a number of pets were sickened or killed by pet food contaminated with the chemical melamine which is used in the manufacture of certain plastics. It is thought that the melamine was added to make a pet food additive appear to have more protein than it really did. Protein content is determined by measuring nitrogen content of food since protein is the main source of nitrogen in food. What is the % by mass of nitrogen in melamine which has the formula C3H6N6?
A) 66.6%
B) 61.2%
C) 11.1%
D) 82.3%
E) Insufficient information is given to find the %N by mass.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What would happen to the amount of HCl produced in the previous question if the amount of H2 is doubled?
A) It would decrease by more than a factor of two.
B) It would decrease by a factor of two.
C) It would remain the same.
D) It would increase by a factor of two.
E) It would increase by more than a factor of two.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
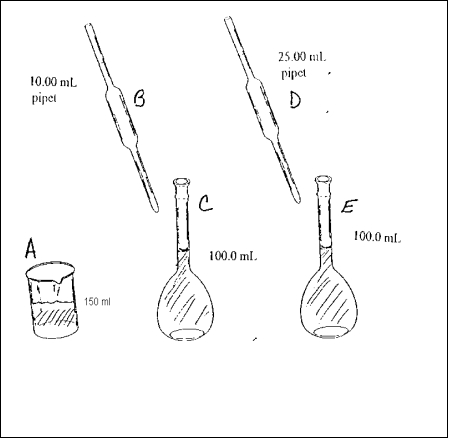 The following four are based on the diagram above which describes the following lab procedure. Ten mL of an initial solution is pipetted into a volumetric flask. The 10.00 mL of initial solution is diluted to 100.0 mL with solvent to form the stock solution. Twenty five mL of the stock solution is then pipetted into another 100.0 mL volumetric flask and diluted to the 100.0 mL mark to form the final solution. The glassware in the diagram has been labeled with letters.
-Which of the following statements correctly describes the relationship between moles of solute in the labeled containers?
The following four are based on the diagram above which describes the following lab procedure. Ten mL of an initial solution is pipetted into a volumetric flask. The 10.00 mL of initial solution is diluted to 100.0 mL with solvent to form the stock solution. Twenty five mL of the stock solution is then pipetted into another 100.0 mL volumetric flask and diluted to the 100.0 mL mark to form the final solution. The glassware in the diagram has been labeled with letters.
-Which of the following statements correctly describes the relationship between moles of solute in the labeled containers?
A) B > A
B) D > E
C) C = D
D) B = C
E) A = C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The thermal decomposition of carbonates leads to the loss of CO2. The decomposition of an unknown carbonate leads to a 35.1% weight loss. The unknown was which of the following compounds.
A) Li2CO3
B) MgCO3
C) CaCO3
D) ZnCO3
E) BaCO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetylene gas, C2H2, burns in the presence of oxygen by the following reaction: 2 C2H2(g) + 5 O2(g) 2 H2O(l) + 4 CO2(g) If 4.5 moles of acetylene gas are to be burned, how many moles of oxygen will be required for the complete reaction of the acetylene?
A) 4.5 moles
B) 23 moles
C) 8.1 moles
D) 5.0 moles
E) none of these
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
When glucose reacts with oxygen in living systems, carbon dioxide and water are produced, and a great deal of energy is liberated. C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O(g) What weight of carbon dioxide can be produced from the reaction of 10.0 grams of glucose with 10.0 grams of oxygen?
A) 2.29 g
B) 2.44 g
C) 13.8 g
D) 14.7 g
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The first silver dollar minted in the Americas was 3.95 cm in diameter and contained 27.07 grams of silver. If the density of silver is 10.5 g/cm3, what was the height of each coin?
A) 0.00791 cm
B) 0.0316 cm
C) 0.0526 cm
D) 0.210 cm
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The density of water in pounds per cubic foot is 62.4 lb/ft3. If a 2 ft3 bag of mulch sold at the local lawn care center weighs 30 lbs, which of the following correctly describes what happens when this mulch is poured on water?
A) The mulch will sink because it is less dense than water.
B) The mulch will sink because it is more dense than water.
C) The mulch will float because it is less dense than water.
D) The mulch will float because it is more dense than water.
E) There is no way of predicting what will happen.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixed oxide of potassium and vanadium is 28.3% by weight potassium and 37.0% by weight vanadium. What is the empirical formula?
A) KV
B) KVO
C) K2V3O
D) K3V3O
E) KVO3
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 126
Related Exams