A) The nucleus is the center region of an atom.
B) The space occupied by the electrons is primarily empty.
C) The nucleus accounts for a small portion of the mass of an atom.
D) The volume occupied by electrons is referred to as an electron cloud.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope of which element is used as the standard for the relative mass scale for atoms?
A) carbon
B) oxygen
C) hydrogen
D) helium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning the molecules depicted is incorrect?
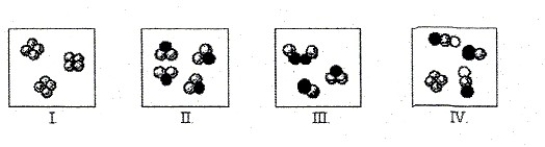
A) The molecules in box I represent a pure substance.
B) The molecules in box III represent a mixture of elements.
C) The molecules in box II represent a compound composed of two different elements.
D) The molecules in box IV represents two different compounds and an element.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of each element are present in 3 formula units of (NH4) 2HAsO4?
A) 10 N atoms, 18 H atoms, 5 As atoms, 18 O atoms
B) 6 N atoms, 9 H atoms, 3 As atoms, 12 O atoms
C) 5 N atoms, 45 H atoms, 4 As atoms, 18 O atoms
D) 6 N atoms, 27 H atoms, 3 As atoms, 12 O atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not a part of atomic theory?
A) Chemical change involves a union, separation, or rearrangement of atoms.
B) A pure substance will have a varying relative number of atom types.
C) All matter is made up of atoms.
D) Atoms are considered indestructible during chemical change.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following properties for the most abundant isotope of an element would not be possessed by the second most abundant isotope of the same element?
A) boiling point is 2403 °C
B) atomic number is 31
C) isotopic mass is 69.92 amu
D) reacts with chlorine to give the compound XCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following compounds does one molecule of the compound contain 3 elements and 8 atoms?
A) NaHCO3
B) C2H7N
C) H3AsO4
D) POCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place in the blank the letter of the correct response in each horizontal row of choices. -________ Mass number of an isotope containing 45 protons and 58 neutrons
A) 13
B) 45
C) 58
D) 103
Correct Answer

verified
Correct Answer
verified
Essay
Determine the relative mass for the hypothetical elements Zp and Wq given the following information: The element Xu has an atomic mass of 24.00 jigs. The element Wq is three times lighter than Xu. The element Zp is two and a half times heavier than a Xu.
Correct Answer

verified
Wq atomic mass is 8.000 jigs
Zp atomic mass is 60.00 jigs
Correct Answer
verified
Multiple Choice
For each description on the left select the correct atom from the response list on the right. Responses may be used more than once or need not be used at all. -________ Has more neutrons than electrons
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following pairs of formulas do the two members of the pair contain the same number of elements as well as the same number of atoms?
A) Co and CO
B) NaHCO3 and NaHSO4
C) SO3 and SO2
D) Cl2CO and C2HCl
Correct Answer

verified
Correct Answer
verified
Short Answer
Fill in the blanks in the following sentences using words from the following response list:
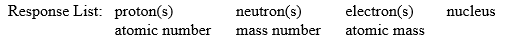 Items in the response list may be used more than once or need not be used at all.
-The mass number for an atom equals the sum of the __________ and __________ in the atom's nucleus.
Items in the response list may be used more than once or need not be used at all.
-The mass number for an atom equals the sum of the __________ and __________ in the atom's nucleus.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the atomic number of an isobar of
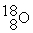 In which there is an equal number of all three types of subatomic particles present?
In which there is an equal number of all three types of subatomic particles present?
A) 8
B) 9
C) 10
D) 11
Correct Answer

verified
B
Correct Answer
verified
Short Answer
Fill in the blanks in the following sentences using words from the following response list:
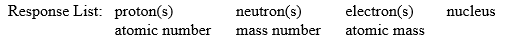 Items in the response list may be used more than once or need not be used at all.
-The mass of a __________ (a neutral species) is many times greater than the mass of an electron and is approximately the same as the mass of a __________.
Items in the response list may be used more than once or need not be used at all.
-The mass of a __________ (a neutral species) is many times greater than the mass of an electron and is approximately the same as the mass of a __________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of hydrogen atoms present in 6 formula units of Mg(C2H3O2) 2?
A) 12
B) 6
C) 36
D) 24
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The diameters and masses of atoms are, respectively, on the order of ________.
A) 10-11 m and 10-11 g
B) 10-23 m and 10-10 g
C) 10-24 m and 10-10 g
D) 10-10 m and 10-23 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place in the blank the letter of the correct response in each horizontal row of choices. -________ Number of elements in the compound Al2SO4
A) 3
B) 4
C) 5
D) 7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which formula is correct for a molecule with 4 P atoms, and 10 O atoms?
A) 3 P4O10
B) 2(P2O3) 2
C) P4O10
D) 3 P3O6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chlorine, which exists in nature in two isotopic forms, has an atomic mass of 35.5 amu. This means that ________.
A) all chlorine atoms have masses of 35.5 amu
B) 35.5 amu is the upper limit for the mass of a chlorine atom
C) 35Cl and 37Cl each constitute approximately 50% of all chlorine atoms
D) 35Cl constitutes 75% of full chlorine atoms, 37Cl represents 25%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements about molecules is correct?
A) Some compounds have molecules as their basic structural unit.
B) For a molecular compound, the atom is the limit of chemical subdivision.
C) For a molecular compound, the atom is the limit of physical subdivision.
D) Molecules of compounds may be either heteroatomic or homoatomic.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 87
Related Exams