A) 0.30
B) 0.60
C) 1.2
D) 2.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of water may be considered pure?
A) bottled water
B) filtered water
C) distilled water
D) chlorinated water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"Hard" water in parts of the Midwest may have a calcium ion concentration as high as 400 ppm.What is this calcium ion concentration when expressed as a percentage?
A) 40%
B) 4%
C) 0.4%
D) 0.04%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The numbers 1 through 4 are used to identify four different elements on the periodic table.Which element is expected to have 2+ charge when it forms an ion? 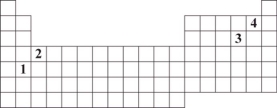
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which correctly describes groundwater?
A) any water found above ground in lakes and rivers;usually needs treatment to remove harmful contaminants
B) any water found above ground in lakes and rivers;usually free of harmful contaminants
C) any water taken from aquifers
D) none of the above.
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The drawing shows a simple way of purifying salt water.What is this process called? 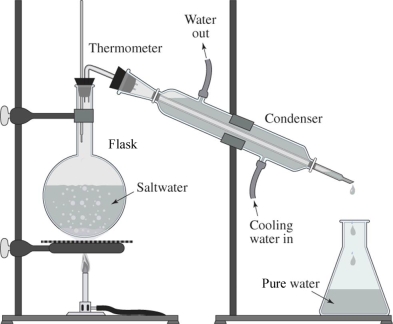
A) filtration
B) distillation
C) ozonation
D) chlorination
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not a consequence of hydrogen bonding between water molecules?
A) Water has a high boiling point.
B) Ice floats on water.
C) Water has a high specific heat.
D) Water is colorless and odorless.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound should be the most soluble in water?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water has one of the highest specific heats of any known liquid.This means that the temperature of a water sample _____ with the input of a _____ amount of energy.
A) decreases greatly;small
B) decreases only slightly;large
C) increases only slightly;large
D) increases greatly;small
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 4-L sample of water contains 80 µg of lead.What is this lead concentration,in parts per billion (ppb) ?
A) 20
B) 80
C) 320
D) 500
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct formula (including charge) of a nitrate ion?
A) NO2+
B) NO2¯
C) NO3¯
D) NO3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ions,if present in drinking water,would pose a significant health risk? i.Pb2+ II.Hg2+ III.Cd2+ IV.Ca2+
A) I,II,and III only
B) II,III,and IV only
C) I,III,and IV only
D) I,II,III,and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In general,hydrogen bonds are ____ and _____ typical covalent bonds.
A) longer than;about one tenth as strong as
B) shorter than;about one tenth as strong as
C) longer than;about ten times as strong as
D) shorter than;about ten times as strong as
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is water a liquid a room temperature instead of a gas?
A) It is a gas a room temperature.
B) Water has hydrogen bonds between the molecules.
C) Water is very acidic.
D) Water is a good solvent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A disadvantage of ozonation over chlorination is
A) the higher cost of ozonation.
B) ozonation does not protect the water after the initial process is complete.
C) the higher cost of ozonation and ozonation does not protect the water after the initial process is complete.
D) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is not true?
A) In forming a solution,the solute dissolves other substances that are called solvents.
B) Water is the solvent in an aqueous solution.
C) Pure water does not conduct electricity.
D) Electrolytes dissolved in water produce a solution that can conduct electricity.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is not a trihalomethane?
A) CHCl3
B) CH3Cl
C) CHF3
D) CHBr2Cl
Correct Answer

verified
Correct Answer
verified
True/False
The MCL for drinking water is the legal limit for the concentration of a contaminant expressed in ppm or ppb.This is always a lower concentration than the MCLG value for the same contaminant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Mixing which of the following will produce a precipitation reaction (give an insoluble product) ?
A) HNO3 (aq) and Sr(OH) 2(aq)
B) LiNO3 and NaI
C) Na2SO4(aq) and Ba(OH) 2(aq)
D) NaOH and KBr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which best symbolizes the hydrogen bonding between two water molecules? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
D
Correct Answer
verified
Showing 1 - 20 of 74
Related Exams