Correct Answer

verified
Correct Answer
verified
Multiple Choice
3.25 kcal is the same amount of energy as ________.
A) 3.25 J
B) 0.777 J
C) 777 J
D) 13600 J
E) 13.6 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many calories are required to raise the temperature of a 150.g sample of gold from 25 °C to 175 °C? The specific heat of gold is 0.0308 cal/g °C.
A) 4.62 cal
B) 116 cal
C) 22500 cal
D) 693 cal
E) 130.cal
Correct Answer

verified
D
Correct Answer
verified
Short Answer
Bromine 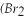 )has a freezing point of -7 °C,and a boiling point of 60 °C.
Indicate the state present or the change of state occurring at each temperature.
--15 °C
)has a freezing point of -7 °C,and a boiling point of 60 °C.
Indicate the state present or the change of state occurring at each temperature.
--15 °C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a chemical change?
A) cutting a rope
B) bending a steel rod
C) making a snowman
D) burning sugar
E) melting gold
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
In a gas,the distance between the particles is ________.
A) very close relative to the size of the molecules
B) close relative to the size of the molecules
C) fixed relative to the size of the molecules
D) small relative to the size of the molecules
E) very large relative to the size of the molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phrase "ability to do work" is a definition of ________.
A) specific heat
B) energy
C) calorie
D) heating
E) cooling
Correct Answer

verified
Correct Answer
verified
True/False
Water freezes at 100 °C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following quantities is not required to calculate the amount of heat energy required to heat water from 25 °C to 55 °C?
A) the mass of the water sample
B) the initial temperature
C) the final temperature
D) the specific heat of water
E) the heat of vaporization for water
Correct Answer

verified
Correct Answer
verified
True/False
The specific heat has units of kcal.
Correct Answer

verified
Correct Answer
verified
True/False
The heat of fusion of water is larger than the heat of vaporization.
Correct Answer

verified
Correct Answer
verified
True/False
Kinetic energy is the energy of motion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Coins in a piggy bank are an example of a(n) ________.
A) compound
B) heterogeneous mixture
C) element
D) homogeneous mixture
E) none of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The heat of fusion is the amount of heat necessary to change one gram of a substance from the solid to the ________ state at its melting point.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy stored in molecules is ________.
A) specific heat
B) kinetic energy
C) potential energy
D) work
E) a calorie
Correct Answer

verified
Correct Answer
verified
Short Answer
Liquid water changing to ice is an example of a ________ change.
Correct Answer

verified
physical
Correct Answer
verified
True/False
One gram of fat has less energy that one gram of protein.
Correct Answer

verified
Correct Answer
verified
Short Answer
A temperature of 50.°F is equivalent to ________°C.
Correct Answer

verified
Correct Answer
verified
Short Answer
The ________ state of matter has a constant shape and volume.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Climate change is believed to result from all of the following except ________.
A) burning of fossil fuels
B) increasing levels of carbon dioxide in the atmosphere
C) deforestation
D) movement of the earth closer to the sun
E) carbon dioxide trapping the heat produced by the sun
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 108
Related Exams