Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following question(s) ,consider the following equation.
2Mg + 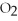 → 2MgO
-How many grams of MgO are produced when 40.0 grams of
→ 2MgO
-How many grams of MgO are produced when 40.0 grams of 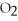 react completely with Mg?
react completely with Mg?
A) 40.3 g
B) 50.4 g
C) 60.5 g
D) 101 g
E) 202 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction takes place when an electric current is passed through water.It is an example of a ________ reaction.
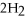 O
O 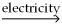 2
2 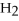 +
+ 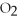
A) combination
B) single replacement
C) combustion
D) decomposition
E) double replacement
Correct Answer

verified
Correct Answer
verified
Short Answer
What type of reaction is the following? 2KClO3 → 2KCl + 3O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of copper(II) sulfate,CuSO4 ?
A) 16.00 g/mole
B) 63.60 g/mole
C) 111.6 g/mole
D) 159.6 g/mole
E) 319.2 g/mole
Correct Answer

verified
Correct Answer
verified
Short Answer
The molar mass of copper(II)nitrate,Cu(N 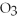
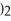 is ________.
is ________.
Correct Answer

verified
Correct Answer
verified
True/False
The following reaction is correctly balanced.
KCl 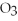 → KCl +
→ KCl + 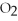
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction that releases energy as it occurs is classified as a(n) ________.
A) endothermic reaction
B) exothermic reaction
C) oxidation-reduction reaction
D) catalyzed reaction
E) decomposition reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following question(s) ,consider the following balanced equation.
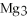
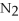 + 6
+ 6 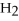 O → 3
O → 3 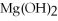 + 2
+ 2 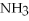 -How many grams of
-How many grams of 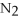 are produced when 125 g of
are produced when 125 g of 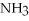 reacts as shown in the following balanced equation?
4
reacts as shown in the following balanced equation?
4 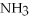 + 6NO → 5
+ 6NO → 5  + 6
+ 6  O
O
A) 206 g
B) 257 g
C) 165 g
D) 7.35 g
E) 145 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Answer the question(s) below about the following reaction.
2 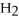
 → 2
→ 2 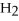 O +
O + 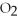 -How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2) produce,if decomposition is complete?
-How many moles of oxygen gas can 5.0 moles of hydrogen peroxide (H2O2) produce,if decomposition is complete?
A) 5.0 moles
B) 2.5 moles
C) 1.0 mole
D) 2.0 moles
E) 10.moles
Correct Answer

verified
Correct Answer
verified
Short Answer
Acetylene gas, 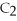
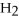 ,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
2
,reacts with oxygen according to the following equation.If 1 mole of acetylene react completely with sufficient oxygen,how many moles of carbon dioxide are produced?
2 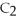
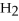 + 5
+ 5 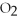 → 4C
→ 4C 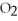 +
+  O
O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following question(s) ,consider the following balanced equation.
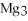
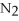 + 6
+ 6 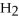 O → 3
O → 3 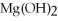 + 2
+ 2 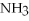 -When 2 moles of
-When 2 moles of 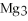
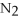 are allowed to react,how many moles of
are allowed to react,how many moles of 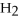 O also react?
O also react?
A) 1 mole
B) 4 moles
C) 6 moles
D) 8 moles
E) 12 moles
Correct Answer

verified
Correct Answer
verified
Short Answer
What type of reaction is the following?
Zn + 2HCl → Zn 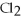 +
+ 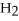
Correct Answer

verified
Correct Answer
verified
True/False
The following is a decomposition reaction. 2KClO3 → 2KCl + 3O2
Correct Answer

verified
Correct Answer
verified
True/False
In the reaction of hydrogen gas with oxygen gas to produce water,1 mole of oxygen gas can produce 2 moles of water,given sufficient hydrogen available.
Correct Answer

verified
Correct Answer
verified
True/False
Avogadro's number is the number of grams in a mole.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ________ is the minimum energy needed for a chemical reaction to begin.
A) reaction energy
B) activation energy
C) energy of reactants
D) energy of products
E) heat of reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of 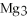
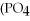
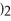 ,a substance formerly used in medicine as an antacid?
,a substance formerly used in medicine as an antacid?
A) 71.28 g/mole
B) 118.3 g/mole
C) 150.3 g/mole
D) 214.3 g/mole
E) 262.9 g/mole
Correct Answer

verified
Correct Answer
verified
True/False
In the following reaction C is reduced.
2C + 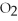 → 2CO
→ 2CO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ________ is the energy difference between reactants and products in a chemical reaction.
A) transition energy
B) activation energy
C) product energy
D) overall energy
E) heat of reaction
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 109
Related Exams