A) 800.g
B) 0.0050 g
C) 8.0 g
D) 2.0 g
E) 200.g
Correct Answer

verified
Correct Answer
verified
Short Answer
Polar solutes are soluble in ________ solvents.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration,in mass percent (m/m) ,of a solution prepared from 50.0 g NaCl and 150.0 g of water?
A) 0.250% (m/m)
B) 33.3% (m/m)
C) 40.0% (m/m)
D) 25.0% (m/m)
E) 3.00% (m/m)
Correct Answer

verified
Correct Answer
verified
Short Answer
When MgO is added to water,the salt will be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetic acid can be classified as a ________.
A) gas
B) solid
C) weak electrolyte
D) strong electrolyte
E) ionic compound
Correct Answer

verified
Correct Answer
verified
Short Answer
A substance that produces only a small number of ions in solution is known as a ________ electrolyte.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molarity of a solution of 5.0 g of KCl in 100.mL of solution is ________.
A) 0.038 M
B) 0.067 M
C) 0.67 M
D) 0.13 M
E) 1.3 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Henry's law,the solubility of a gas in a liquid ________.
A) decreases as the gas pressure above the liquid increases
B) increases as the gas pressure above the liquid increases
C) remains the same as the temperature increases
D) depends on the liquid polarity
E) depends on the liquid density
Correct Answer

verified
Correct Answer
verified
Short Answer
A substance that completely ionizes in water is a ________ electrolyte.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A ________ will pass through a filter but not a semipermeable membrane.
A) solid
B) precipitate
C) solution
D) suspension
E) colloid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the new mass/volume percent (m/v) of a KOH solution that is prepared by diluting 110 mL of a 6.0% (m/v) KOH solution to 330 mL?
A) 2.0% (m/v)
B) 1.0% (m/v)
C) 6.0% (m/v)
D) 12% (m/v)
E) 18% (m/v)
Correct Answer

verified
Correct Answer
verified
Matching
Indicate whether each of the following compounds dissolves in water to give ions,molecules,or both.
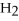
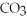 ,a weak electrolyte
,a weak electrolyte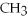
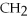 OH,a nonelectrolyte
OH,a nonelectrolyte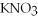 ,a soluble salt
,a soluble saltCorrect Answer
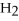
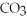 ,a weak electrolyte
,a weak electrolyte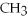
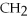 OH,a nonelectrolyte
OH,a nonelectrolyte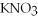 ,a soluble salt
,a soluble salt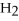
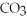 ,a weak electrolyte
,a weak electrolyte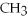
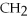 OH,a nonelectrolyte
OH,a nonelectrolyte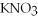 ,a soluble salt
,a soluble saltMultiple Choice
Which of the following molecules can form hydrogen bonds?
A) ![]()
B) NaH
C) ![]()
D) ![]()
E) HI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molarity of a KCl solution made by diluting 75.0 mL of a 0.200 M solution to a final volume of 100.mL?
A) 0.267 M
B) 0.150 M
C) 0.200 M
D) 6.67 M
E) 0.100 M
Correct Answer

verified
Correct Answer
verified
Short Answer
A substance that carries an electric current when dissolved in water is called a(n)________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When KCl dissolves in water ________.
A) the ![]() ions are attracted to dissolved
ions are attracted to dissolved ![]() ions
ions
B) the ![]() ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
C) the ![]() ions are attracted to
ions are attracted to ![]() ions on the KCl crystal
ions on the KCl crystal
D) the ![]() ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
E) the ![]() ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of moles of a compound dissolved in one liter of a solution is called the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An increase in the temperature of a solution usually ________.
A) increases the boiling point
B) increases the solubility of a gas in the solution
C) increases the solubility of a solid solute in the solution
D) decreases the solubility of a solid solute in the solution
E) decreases the solubility of a liquid solute in the solution
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which solution is isotonic to a red blood cell?
A) water
B) 0.5% NaCl
C) 2% glucose
D) 0.9% NaCl
E) 10% glucose
Correct Answer

verified
Correct Answer
verified
Short Answer
________ can pass through filters but cannot pass through semipermeable membranes.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 78
Related Exams