A) 10450
B) 0.598
C) 1.05
D) 10.5
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
In physical changes,the atoms or molecules that compose the matter do not change their identity,even though the matter may change its appearance.
Correct Answer

verified
Correct Answer
verified
True/False
Saltwater is a homogeneous mixture.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many joules are there in a 255 calorie snack bar?
A) 2.55 × 105
B) 1.07 × 106
C) 1.07 × 103
D) 6.09 × 104
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following can be considered physical properties EXCEPT:
A) taste.
B) color.
C) flammability.
D) density.
E) boiling point.
Correct Answer

verified
Correct Answer
verified
True/False
Flammability of gasoline is a chemical property.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a technique that could be used to separate a mixture into its components?
A) stirring
B) decanting
C) filtration
D) distillation
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which state of matter has atomic spacing that is close together and definite shape?
A) liquid
B) solid
C) gas
D) plasma
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many kilojoules are there in 95.0 Calories?
A) 2.27 × 107
B) 3.97 × 10-4
C) 397
D) 22.7
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The corrosion of iron is a physical change.
Correct Answer

verified
Correct Answer
verified
True/False
If a pure material decomposes when heated into simpler substances,this proves that the material was a compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the specific heat (J/g∙°C) of a metal object whose temperature increases by 3.0°C when 17.5 g of metal was heated with 38.5 J?
A) 4.18
B) 0.15
C) 0.73
D) 1.4
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
When a chemical "cold pack" is activated,the chemical reactants absorb heat from the surroundings.
Correct Answer

verified
Correct Answer
verified
True/False
Liquid and gas molecules can easily be compressed,while in a solid the molecules are incompressible.
Correct Answer

verified
Correct Answer
verified
True/False
An energy diagram that shows the products having higher energy than the reactants illustrates an endothermic reaction.
Correct Answer

verified
Correct Answer
verified
True/False
When a cold ice cube is dropped into a warm cup of water,energy is transferred as heat from the ice to the water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a particular process is endothermic,the reverse process must be a (an) :
A) chemical change.
B) isothermal process.
C) exothermic process.
D) endothermic process.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the table of specific heat values below,what is the identity of a 26.2 g metal sample that increases by 8.5°C when 100.0 J of energy is absorbed?
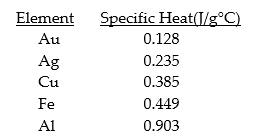
A) Fe
B) Al
C) Au
D) Ag
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A moving bowling ball has kinetic energy.
Correct Answer

verified
Correct Answer
verified
True/False
Matter can be destroyed in a combustion reaction (such as burning fuel).
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 118
Related Exams