A) ancient Greek philosopher who proposed that matter was continuous
B) created the modern periodic table
C) proposed the modern Atomic Theory
D) discovered the existence of electrons
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
John Dalton was the first person recorded as thinking that matter was ultimately composed of atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fictional element has two isotopes,each making up 50% of the population.Isotope 1 has a mass of 80.0 amu,Isotope 2 has a mass of 85.0 amu.Calculate the atomic mass of the fictional element.
A) 82.5 amu
B) 42.5 amu
C) 40 amu
D) 165 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
All elements have three or more naturally occurring isotopes.
Correct Answer

verified
Correct Answer
verified
True/False
John Dalton formalized an atomic theory that gained acceptance in the early 19th century.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the nature of electrical charge is FALSE?
A) Electrical charge is a fundamental property of protons and electrons.
B) Positive and negative electrical charges attract each other.
C) Positive-positive or negative-negative charges repel each other.
D) Positive and negative charges cancel each other so that a proton and electron,when paired,are charge neutral.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
True/False
J.J.Thomson discovered the existence of protons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom that has the same number of neutrons as 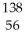 Ba is:
Ba is:
A) ![]() Cs
Cs
B) ![]() Ba
Ba
C) ![]() La
La
D) ![]() Xe
Xe
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a main-group element?
A) Ce
B) Co
C) Cu
D) Cs
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below is NOT consistent with the nuclear theory of the atom as proposed by Rutherford?
A) Most of the atom's mass and all of its positive charge is contained in a small core called the nucleus.
B) Electrical charge is a fundamental property of protons and electrons in which like charges repel and opposite charges attract.
C) Most of the volume of the atom is empty space occupied by tiny,negatively charged electrons.
D) There are as many electrons outside the nucleus as there are protons inside the nucleus in a neutral atom.
E) All of the above statements are consistent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A specific isotope of an element is known to have 15 protons and 16 neutrons.Which symbol would properly represent this isotope?
A) ![]() Ga
Ga
B) ![]() P
P
C) ![]() X
X
D) ![]() S
S
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Main-group elements tend to form ions that have the same number of total electrons as the nearest halogen.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
What is the atomic symbol for silver?
A) S
B) Ag
C) Au
D) Si
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Aluminum is one of the most commonly used metalloids.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
All of the following statements about different elements are true EXCEPT:
A) Barium is an alkaline earth metal.
B) Manganese is a transition metal.
C) Sulfur is considered a metalloid.
D) Krypton is one of the noble gases.
E) Iodine is a halogen.
Correct Answer

verified
C
Correct Answer
verified
True/False
The gold foil experiment proved that large regions of the atoms consisted of empty space.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The only known atom that does not contain a neutron is one of the isotopes of hydrogen.The mass number,symbolized as "A" in the text,of this hydrogen isotope must be:
A) 1.01
B) 0
C) 1
D) 2
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The charges on electrons and neutrons cancel each other to give neutral atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are in Br-?
A) 4
B) 7
C) 34
D) 36
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The smell of the ocean is caused by atoms.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 112
Related Exams