Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of 12.0 M HCl is required to make 75.0 mL of 3.50 M HCl?
A) 21.9 mL
B) 0.560 mL
C) 257 mL
D) 560.mL
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Molality is calculated by dividing grams of solute by kilograms of solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a strong electrolyte?
A) C6H12O6
B) C7H14O5
C) C4H8O2
D) NaC2H3O2
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of NaF are in 34.2 grams of a 45.5% by mass NaF solution?
A) 0.814
B) 75.2
C) 15.6
D) 0.371
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the change in the boiling point of a solution made by dissolving 14.7 g of C6H12O6 into 150.0 ml of water? The density of water is 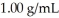 and
and 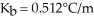 .
.
A) 0.502°C
B) 5.22°C
C) 0.0418°C
D) 0.279°C
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many mL of 0.218 M sodium sulfate react with exactly of BaC given the reaction:
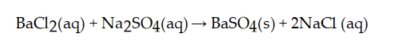
A) 13.1
B) 5.52
C) 24.6
D) 2.86
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the solubility of sodium acetate (Molar mass = 82 g/mol) is 76 grams per 100 grams of water,which of the following solutions would be considered supersaturated?
A) 8.5 moles of sodium acetate dissolved in 1 L of water
B) 5.5 moles of sodium acetate dissolved in 500 mL of water
C) 1.8 moles of sodium acetate dissolved in 300 mL of water
D) 1.2 moles of sodium acetate dissolved in 200 mL of water
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
If 50 grams of salt dissolves into 250 grams of water,the resulting solution must have a mass of 300 grams.
Correct Answer

verified
Correct Answer
verified
True/False
One liter of a 2.0 M NaOH (aq)solution contains the same number of Na+ ions as does one liter of a 1.0 M Na2CO3 (aq)solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is saturated in both nitrogen gas (N2) and sodium iodide (NaI) at 50°C.When the solution is cooled to 25°C,which of the following is most likely to occur?
A) Some nitrogen gas bubbles out of solution.
B) Some sodium iodide will precipitate out of solution.
C) Both A and B will happen.
D) Nothing will happen.
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
A solution is a homogeneous mixture of two or more substances.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of barium sulfate are produced if 25.34mL of 0.113M BaCl2 completely react given the reaction: BaCl2(aq) + Na2SO4 (aq) → BaSO4 (s) + 2NaCl (aq)
A) 5.90
B) 26.3
C) 1039
D) 0.668
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances is NOT a solution?
A) humid air
B) beer
C) oxygen
D) steel
E) All of the above are solutions.
Correct Answer

verified
Correct Answer
verified
True/False
Osmotic pressure is the pressure required to completely reverse the direction of solvent movement in osmosis.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following aqueous solutions would have the higher concentration of K+ (aq) ions? (Assume total solubility in water.)
A) 1.0 M KC2H3O2
B) 1.0 M KNO3
C) 1.0 M K2CO3
D) 1.0 M K3PO4
E) All of these solutions have the same concentration of K+ (aq) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of a 2.18 M solution can be made from 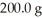
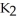 S?
S?
A) 0.832
B) 1.81
C) 0.252
D) 1.20
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What will happen if a healthy red blood cell is placed into a container of pure water?
A) The cell will totally dissolve in the water.
B) The cell will remain unchanged.
C) The cell will become swollen.
D) The cell will shrink.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An osmosis cell is constructed of a "U" shaped tube with a semipermeable membrane separating the two arms of the tube.Suppose a concentrated solution is placed in the left arm of the U-tube,and a dilute concentration of the same substance is poured into the other arm to the same height.After a period of time has elapsed,you would expect to find that:
A) the level of liquid in the left arm is now higher.
B) the level of the liquid in the right arm is now higher.
C) the levels in both arms stay at the same height.
D) no molecules of any type cross the membrane.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following aqueous solutions has the highest molar concentration of Na+ (aq) ? (Assume each compound is fully dissolved in water.)
A) 3.0M NaCl (sodium chloride)
B) 3.0M NaC2H3O2 (sodium acetate)
C) 1.5M Na2SO4 (sodium sulfate)
D) 1.0M Na3PO4 (sodium phosphate)
E) All of these solutions have the same concentration of Na+ (aq) .
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 122
Related Exams