A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of 14C is 5730 yr.Assuming some charcoal from a campfire 29,000 years old was found, what fraction of the original C-14 would remain today?
A) 3.0 × 10−2
B) 0.20
C) 3.5
D) 0.33
E) 0.29
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 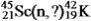
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced.Which particular isotope of this element is formed? 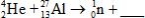
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 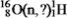
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is used to image the brain?
A) (18O)
B) (131I)
C) (123I)
D) (24Na)
E) (99Tc)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name given to the nuclear process that results from the bombardment of nuclei by neutrons, protons, or other nuclei?
A) Nuclear transmutation
B) Protonation
C) Nucleation
D) Nuclear condensation
E) Radioactivity
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation.U-238 + ? → 1 neutron + Fm-249
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 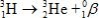
A) 0.029 yr
B) 7.4 yr
C) 9.1 yr
D) 16 yr
E) 35 yr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Beta particles are identical to
A) protons.
B) helium atoms.
C) hydrogen atoms.
D) helium nuclei.
E) electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cesium-134 is a β emitter with a half-life of 2.0 years.How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A) 0.0024 g
B) 0.078 g
C) 0.25 g
D) 0.50 g
E) 80.0 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When atoms of beryllium−9 are bombarded with alpha particles, neutrons are produced.What new isotope is also formed? 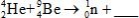
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is an incorrect representation of the indicated particle or nucleus?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What other particle is emitted when a neutron is converted to a proton in a nucleus?
A) Gamma particle
B) Alpha particle
C) Positron
D) Beta particle
E) None of the answers is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What fraction of radioactive atoms remains in a sample after six half-lives?
A) zero
B) 1/6
C) 1/16
D) 1/32
E) 1/64
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction, identify X. 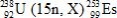
A) 7β
B) 3α
C) 4n
D) ![]()
E) 15p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the equation below, what particle or type of radiation needs to be included to balance the equation? 220Rn → ? + alpha particle
A) Ra-224
B) Rn-224
C) Rn-216
D) At-220
E) Po-216
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the nuclear process called where small nuclei are combined into larger ones?
A) Photonuclear reactions
B) Nuclear fission
C) Thermal conductivity
D) Nuclear combination
E) Nuclear fusion
Correct Answer

verified
Correct Answer
verified
Showing 21 - 38 of 38
Related Exams