A) magnesium-23.
B) sulfur-31.
C) phosphorus-27.
D) silicon-26.
E) aluminum-27.
Correct Answer

verified
Correct Answer
verified
Essay
Complete and balance the nuclear equation 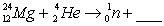 .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Petroleum is a fossil fuel containing many different carbon compounds. If the carbon atoms in petroleum have been in the ground for 100 million years, what fraction of the initial 14C atoms is still there? (t1/2 = 5,370 yr)
A) 0
B) 1 * 10-10
C) 5.7 * 10-5
D) 1.0 * 10-3
E) 5.7 * 10-1
Correct Answer

verified
Correct Answer
verified
Short Answer
Radioactive nitrogen-13 has a half-life of 10 minutes. After 40 minutes, how much of this isotope would remain in a sample that originally contained 96 mg?
Correct Answer

verified
Correct Answer
verified
Essay
Balance the equation 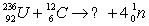
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced. The particular isotope formed is 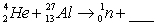
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of 14C is 5,730 yr. Assuming some charcoal from a campfire 29,000 years old was found, what fraction of the original C-14 would remain today?
A) 3.0 * 10-2
B) 0.197
C) 3.51
D) 33.3
E) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction, identify X. 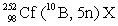
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a radioisotope shows an activity of 999 disintegrations per minute due to beta decay. If after 1.10 years the activity is 952 disintegrations per minute, what is the half-life of this radioisotope?
A) 4.38 * 10-2 yr
B) 11.4 yr
C) 0.25 yr
D) 15.8 yr
E) 9.1 yr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What fraction of radioactive atoms remains in a sample after six half-lives?
A) zero
B) 1/6
C) 1/16
D) 1/32
E) 1/64
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Decay of tin-110 by electron capture yields
A) tin-109.
B) cadmium-106.
C) indium-110.
D) antimony-110.
E) tellurium-114.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The only stable isotope of aluminum is aluminum-27. What type of radioactive decay should be expected from 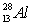 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 101 - 112 of 112
Related Exams