A) picture (a)
B) picture (b)
C) picture (c)
D) picture (d)
Correct Answer

verified
Correct Answer
verified
Short Answer
Using shorthand notation,the ground-state electron configuration for C4- is predicted to be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following four spheres represent a Na atom,a Na+ ion,a Cl atom,and a Cl- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 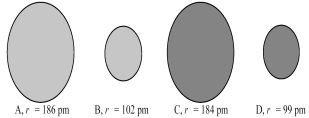
A) A → B + e- and C → D + e-
B) A → B + e- and D → C + e-
C) B → A + e- and C → D + e-
D) B → A + e- and D → C + e-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic compounds would be expected to have the highest lattice energy?
A) LiF
B) LiCl
C) LiBr
D) LiI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkali metal reacts with nitrogen to form a nitride?
A) Li
B) Na
C) K
D) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a solid at room temperature?
A) fluorine
B) chlorine
C) bromine
D) iodine
Correct Answer

verified
Correct Answer
verified
Short Answer
Using shorthand notation,the ground-state electron configuration for Tl+ is predicted to be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
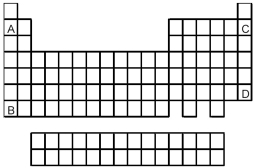 -Atoms of which element,indicated by letter on the periodic table above,would be expected to have the highest first ionization energy,Ei1?
-Atoms of which element,indicated by letter on the periodic table above,would be expected to have the highest first ionization energy,Ei1?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the energy change for the formation of CaF2(s) from its elements in their standard states and the following information: 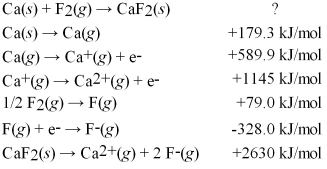
A) +4046 kJ/mol
B) -965 kJ/mol
C) -1214 kJ/mol
D) -3286 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 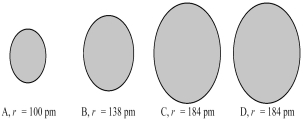 -Which sphere most likely represents the Ca2+ ion?
-Which sphere most likely represents the Ca2+ ion?
A) A
B) B
C) A or B
D) C or D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To reach a noble gas electron configuration how many electrons would sulfur have to adopt?
A) 1
B) 2
C) 6
D) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which liberates the most energy?
A) Br(g) + e⁻ → Br⁻(g)
B) Cl(g) + e⁻ → Cl⁻(g)
C) F(g) + e⁻ → F⁻(g)
D) I(g) → I⁻(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name for the group of elements indicated by the shaded portion of the periodic table? 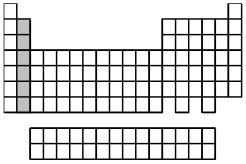
A) alkali metals
B) alkaline earth metals
C) inner-transition metals
D) transition metals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
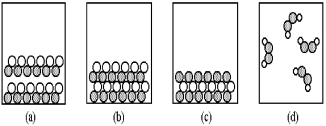 -Which of the above pictures are more likely to represent covalent compounds?
-Which of the above pictures are more likely to represent covalent compounds?
A) pictures (a) and (b)
B) pictures (a) and (d)
C) pictures (b) and (c)
D) pictures (b) and (d)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Interhalogen compounds can be produced by reacting two different halogens together.Which one of the following compounds does not exist?
A) ClF
B) ClBr
C) ClBr3
D) IF3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not a chemical reaction of the alkali metal sodium?
A) 4 Na(s) + 2 O2(g) → 2 Na2O(s) + Na2O2(s)
B) 2 Na(s) + 2 NH3(l) → 2 NaNH2(sol) + H2(g)
C) 2 Na(s) + Cl2(g) → 2 NaCl(s)
D) 2 Na(s) + 2 H2O(l) → 2 NaOH(aq) + H2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
![-Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration [Xe]6s<sup>2</sup> 4f<sup>14</sup> 5d<sup>10</sup>? A) A B) B C) C D) D](https://d2lvgg3v3hfg70.cloudfront.net/TB4939/11ea7a38_c8f8_fecc_aa4c_a1e4ee487bb4_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration [Xe]6s2 4f14 5d10?
-Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration [Xe]6s2 4f14 5d10?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkali metal forms preferentially an oxide rather than a peroxide or superoxide?
A) Li
B) Na
C) K
D) Rb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the lattice energy for NaCl(s) using a Born-Haber cycle and the following information: 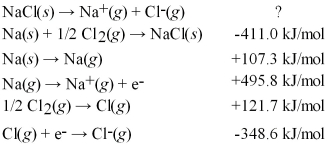
A) +34.8 kJ/mol
B) +690.3 kJ/mol
C) +787.2 kJ/mol
D) +1512 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
![-Which element,indicated by letter on the periodic table above,has a 2+ ion with the electron configuration [Ar]3d<sup>10</sup>? A) A B) B C) C D) D](https://d2lvgg3v3hfg70.cloudfront.net/TB4939/11ea7a38_c8f8_fecc_aa4c_a1e4ee487bb4_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which element,indicated by letter on the periodic table above,has a 2+ ion with the electron configuration [Ar]3d10?
-Which element,indicated by letter on the periodic table above,has a 2+ ion with the electron configuration [Ar]3d10?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 173
Related Exams