A) the system absorbs heat and expands during the process.
B) the system absorbs heat and contracts during the process.
C) the system loses heat and expands during the process.
D) the system loses heat and contracts during the process.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is expected to have the largest carbon-oxygen bond dissociation energy?
A) CO
B) CO2
C) HCO2H
D) CO32-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following can be interpreted as a measure of randomness?
A) enthalpy
B) entropy
C) free energy
D) temperature
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 0.455 g of anthracene,C14H10,is combusted in a bomb calorimeter that has a water jacket containing 500.0 g of water,the temperature of the water increases by 8.63°C.Assuming that the specific heat of water is 4.18 J/(g ∙ °C) ,and that the heat absorption by the calorimeter is negligible,estimate the enthalpy of combustion per mole of anthracene.
A) +39.7 kJ/mol
B) -39.7 kJ/mol
C) -7070 kJ/mol
D) -8120 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is expected when the reaction shown below takes place in a thermally-insulated container outfitted with a movable piston at a constant atmospheric pressure of 1 atm? 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
A) Volume will decrease and work will be done by the system.
B) Volume will decrease and work will be done on the system.
C) Volume will increase and work will be done by the system.
D) volume will decrease and work will be done on the system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The specific heat of copper is 0.385 J/(g ∙°C) .If 34.2 g of copper,initially at 25°C,absorbs 4.689 kJ,what will be the final temperature of the copper?
A) 25.4°C
B) 27.8°C
C) 356°C
D) 381°C
Correct Answer

verified
Correct Answer
verified
Short Answer
Heat transfer measured in a coffee-cup calorimeter at constant pressure is a measure of ________,but heat transfer measured in a bomb calorimeter at constant volume is a measure of ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the sign of ΔS° for each of the following: I.The mixing of two gases at a given temperature and pressure II.C(s) + 2 H2O(g) → CO2(g) + 2 H2(g)
A) ΔS° is negative for I and negative for II.
B) ΔS° is negative for I and positive for II.
C) ΔS° is positive for I and negative for II.
D) ΔS° is positive for I and positive for II.
Correct Answer

verified
Correct Answer
verified
Short Answer
The sign of ΔS° for reaction below is expected to be ________. 2 H2(g)+ O2(g)→ 2 H2O(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following drawing is a representation of the exothermic reaction in which ozone forms dioxygen. 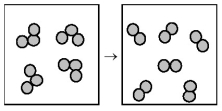 -What are the signs of ΔH and ΔS for this reaction?
-What are the signs of ΔH and ΔS for this reaction?
A) ΔH = +,ΔS = +
B) ΔH = +,ΔS = -
C) ΔH = -,ΔS = +
D) ΔH = -,ΔS = -
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Under thermodynamic standard state conditions the element oxygen occurs as
A) O(g)
B) O2(g)
C) O2(l)
D) O3(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of CH4(g) ,C2H2(g) ,and CH3OH(l) provides the most energy per gram upon combustion and which provides the least? CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l) ΔH° = -890 kJ 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(l) ΔH° = -2599 kJ 2 CH3OH(l) + 3 O2(g) → 2 CO2(g) + 4 H2O(l) ΔH° = -1453 kJ
A) C2H2 provides the most energy per gram and CH4 the least.
B) C2H2 provides the most energy per gram and CH3OH the least.
C) CH4 provides the most energy per gram and CH3OH the least.
D) CH4 provides the most energy per gram and C2H2 the least.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 1.00 mol of benzene is vaporized at a constant pressure of 1.00 atm and at its normal boiling point of 80.1°C,33.9 kJ are absorbed and PΔV for the vaporization process is equal to 2.90 kJ,then
A) ΔE = 31.0 kJ and ΔH = 33.9 kJ.
B) ΔE = 36.8 kJ and ΔH = 33.9 kJ.
C) ΔE = 33.9 kJ and ΔH = 31.0 kJ.
D) ΔE = 33.9 kJ and ΔH = 36.8 kJ.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the work energy,w,gained or lost by the system when a gas expands from 15 L to 35 L against a constant external pressure of 1.5 atm.[1 L ∙ atm = 101 J]
A) -5.3 kJ
B) -3.0 kJ
C) +3.0 kJ
D) +5.3 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25°C the heat of fusion of aluminum is 10.6 kJ/mol and the heat of sublimation is 326.4 kJ/mol.What is the heat of vaporization of aluminum at 25°C?
A) 158.2 kJ/mol
B) 168.5 kJ/mol
C) 315.8 kJ/mol
D) 337.0 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the expansion of an ideal gas into a vacuum at constant temperature,ΔH = 0.What can be said about ΔE and ΔS?
A) ΔE is negative and ΔS is positive.
B) ΔE is zero and ΔS is positive.
C) ΔE is negative and ΔS is zero.
D) ΔE is positive and ΔS is negative.
Correct Answer

verified
Correct Answer
verified
Short Answer
Because the number of moles of gas are increasing from 6 to 7 in the reaction shown below,at constant pressure ΔE is predicted to be slightly ________ negative than ΔH. C3H8(g)+ 5 O2(g)→ 3 CO2(g)+ 4 H2O(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It takes 11.2 kJ of energy to raise the temperature of 145 g of benzene from 25.0°C to 70.0°C.What is the specific heat of benzene?
A) 1) 10 J/(g ∙ °C)
B) 1) 72 J/(g ∙ °C)
C) 3) 48 J/(g ∙ °C)
D) 5) 41 J/(g ∙ °C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enthalpy of fusion of naphthalene,C10H8,is 19.1 kJ/mol at 78.2°C,its melting point.Calculate the entropy of fusion at the melting point.
A) ΔS°fus = 244 J/(K ∙ mol)
B) ΔS°fus = 54.4 J/(K ∙ mol)
C) ΔS°fus = 1.49 J/(K ∙ mol)
D) ΔS°fus = -1.49 J/(K ∙ mol)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant pressure,the combustion of 5.00 g of C2H6(g) releases 259 kJ of heat.What is ΔH for the reaction given below? 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(l)
A) -43.2 kJ
B) -779 kJ
C) -1560 kJ
D) -3120 kJ
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 163
Related Exams