A) 0) 30
B) 3) 18
C) 3) 76
D) 4) 03
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the Ksp for silver sulfite if the solubility of Ag2SO3 in pure water is 4.6 × 10-3 g/L.
A) 3) 8 × 10-15
B) 1) 5 × 10-14
C) 2) 4 × 10-10
D) 4) 8 × 10-10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH. 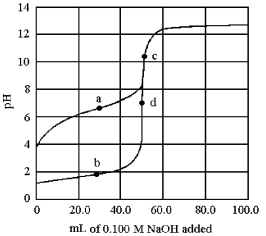 -Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
-Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
A) points a and b
B) points a and c
C) points b and d
D) points c and d
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the Ka of the amino acid glycine if it is 75.0% dissociated at pH = 10.08?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar solubility of thallium(I) chloride in 0.40 M NaCl at 25°C.Ksp for TlCl is 1) 7 × 10-4.
A) 6) 8 × 10-5 M
B) 4) 2 × 10- 4 M
C) 8) 2 × 10-3 M
D) 1) 3 × 10-2 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is a net ionic equation for the neutralization reaction of a weak acid with a weak base?
A) H3O+(aq) + OH-(aq) ⇌ 2 H2O(l)
B) HF(aq) + NH3(aq) ⇌ NH4+(aq) + F-(aq)
C) HF(aq) + OH-(aq) ⇌ H2O(l) + F-(aq)
D) H3O+(aq) + NH3(aq) ⇌ NH4+(aq) + H2O(l)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 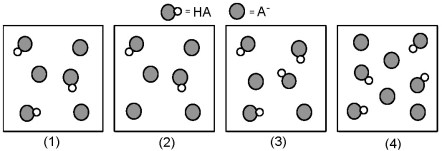 -Which solution has the greatest buffer capacity?
-Which solution has the greatest buffer capacity?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these neutralization reactions has a pH < 7 when equal molar amounts of acid and base are mixed?
A) CH3CO2H(aq) + NaOH(aq) ⇌ H2O(l) + NaCH3CO2(aq)
B) HCl(aq) + C5H5N(aq) ⇌ C5H5NHCl(aq)
C) HCl(aq) + NaOH(aq) ⇌ H2O(l) + NaCl(aq)
D) H2SO4(aq) + 2 KOH(aq) ⇌2 H2O(l) + K2SO4(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the [CH3CO2-]/[CH3CO2H] ratio necessary to make a buffer solution with a pH of 4.44? Ka = 1.8 × 10-5 for CH3CO2H.
A) 0) 50:1
B) 0) 94:1
C) 1) 1:1
D) 2) 0:1
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the pH of the solution formed when 50 mL of 0.250 M NaOH is added to 50 mL of 0.120 M HCl?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 50 mL of 0.10 M NaF is added to 50 mL of 0.10 M HF,relative to the pH of the 0.10 M HF solution the pH of the resulting solution will
A) become 7.
B) decrease.
C) increase.
D) remain the same.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 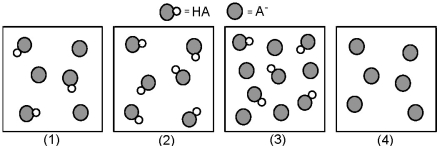 -Which of the solutions are buffer solutions?
-Which of the solutions are buffer solutions?
A) (1) and (2)
B) (1) and (3)
C) (2) and (3)
D) (2) and (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a buffer solution made by mixing 50.0 mL of 0.100 M potassium hydrogen phthalate with 13.6 mL of 0.100 M NaOH and diluting the mixture to 100.0 mL with water? The Ka2 for hydrogen phthalate is 3.1 × 10-6.
A) 3) 25
B) 5) 08
C) 5) 51
D) 5) 94
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Henderson-Hasselbalch equation for the acidic buffer HA/A-?
A) pH = -log[H3O+]
B) pH = 14 - pOH
C) pH = pKa + log{[A-]/[HA]}
D) pH = pKa - log{[A-]/[HA]}
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a solution made by mixing 10.00 mL of 0.10 M acetic acid with 10.00 mL of 0.10 M KOH? Assume that the volumes of the solutions are additive.Ka =1.8 × 10-5 for CH3CO2H.
A) 5) 28
B) 7) 00
C) 8) 72
D) 10.02
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. )  -Which solution has the highest pH?
-Which solution has the highest pH?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution may contain the following ions Ag+,Cu2+,Cd2+,Mn2+,Ni2+ and Na+.A white precipitate formed when 0.10 M NaCl was added and after this was removed the solution was treated with H2S gas under acidic conditions and no precipitate formed.When the solution was made basic and again treated with H2S gas a dark colored precipitate formed.If no further tests were made then what conclusions can you draw?
A) possible ions present Ag+,Mn2+,Ni2+
B) possible ions present Ag+,Mn2+,Ni2+,Na+
C) possible ions present Ag+,Cu2+,Cd2+
D) possible ions present Ag+,Cu2+,Cd2+,Na+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 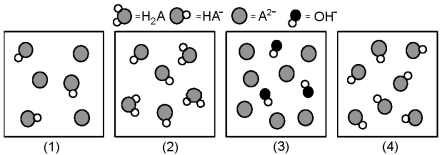 -Which picture represents the system halfway between the first and second equivalence points?
-Which picture represents the system halfway between the first and second equivalence points?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
TRIS {(HOCH2) 3CNH2} is one of the most common buffers used in biochemistry.A solution is prepared by adding enough TRIS and 12 M HCl(aq) to give 1.00 L of solution with [TRIS] = 0.30 M and [TRISH+] = 0.60 M.What is the pH of this buffered system if the pKb is 5.92?
A) 5) 92
B) 6) 22
C) 7) 78
D) 8) 08
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 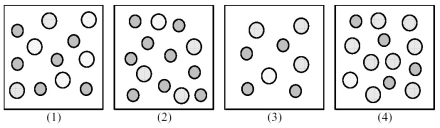 -If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of NH3 is added and equilibrium is restored?
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of NH3 is added and equilibrium is restored?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 190
Related Exams