A) Ca(NO2) 2
B) Ca3N2
C) Ca2(NO2) 2
D) Ca2N3
E) Ca(NO2) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following lists gives the correct symbols for the elements phosphorus,potassium,silver,chlorine,and sulfur?
A) P,Po,Ag,Cl,S
B) K,Ag,Po,Cl,S
C) P,K,Ag,Cl,S
D) Ph,K,Ag,S,Cl
E) Ph,Po,Ag,Cl,S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for Zn2+ is
A) monozinc ion.
B) zinc ion.
C) zinc(2) ion.
D) zinc(I) ion.
E) zinc.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium has two naturally occurring isotopes,6Li and 7Li .The average atomic mass of lithium is 6.941.Which of the following statements concerning the relative abundance of each isotope is correct?
A) The abundance of 7Li is greater than 6Li.
B) The abundance of 7Li is less than 6Li.
C) The abundance of 6Li is equal to the abundance of 7Li.
D) Not enough data is provided to determine the correct answer.
E) Based on the atomic mass,only 7Li occurs naturally.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for manganese(III) oxide?
A) MnO
B) Mn2O
C) Mn3O2
D) Mn2O3
E) MnO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the atomic symbol for the element cobalt?
A) CO
B) Co
C) C
D) co
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the largest atomic mass?
A) hafnium
B) nickel
C) mercury
D) argon
E) carbon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.0-g sample of washing soda,Na2CO3 • 10H2O,has  carbon atoms.How many oxygen atoms are present in 2.0g of washing soda?
carbon atoms.How many oxygen atoms are present in 2.0g of washing soda?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nuclides contains more protons than neutrons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Energy from the following reaction provided the lift for the moon lander: __ (CH3) 2N2H2 + __ N2O4 → __ N2 + __ H2O + __ CO2 When the equation is balanced,the smallest whole-number coefficient of nitrogen is
A) 5.
B) 4.
C) 1.
D) 3.
E) 2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complete combustion of propane,C3H8,yields carbon dioxide and water: - C3 H8 + - O2→ - CO2 + - H2O The smallest whole-number coefficient of oxygen in the balanced equation is
A) 6.
B) 3.
C) 7.
D) 4.
E) 5.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following equations is not balanced?
A) 2Sb2OS2 + 10O2 → 2Sb2O5 + 4SO3
B) (NH4) 2Cr2O7 → N2 + 4H2O + Cr2O3
C) C12H22O11 + 12O2 → 12CO2 + 11H2O
D) 2NaCl + Pb(NO3) 2 → PbCl2 + 2NaNO3
E) Fe3O4 + 3CO → 3Fe + 3CO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formulas of the carbonate ion,the ammonium ion,and the chlorate ion are represented,respectively,as
A) CO32-,NH2-,ClO3-.
B) CO32-,NH4+,ClO3-.
C) CO2-,NH4+,ClO-.
D) P3-,NH3+,ClO2-.
E) CO32-,NH3+,ClO2-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The chemical name for the binary,non-ionic molecule with the formula HI is
A) hydrogen iodide.
B) monohydrogen iodide.
C) hydride iodide.
D) hydrogen iodine.
E) monohydrogen iodine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs of compounds can be used to illustrate the law of multiple proportions?
A) H2O and HCl
B) NO and NO2
C) NH4 and NH4Cl
D) ZnO2 and ZnCl2
E) CH4 and CO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A subatomic particle is
A) a piece of an atom.
B) only found in the nucleus of an atom.
C) always positively charged.
D) larger than the nucleus of an atom.
E) always negatively charged.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the Thomson model of the atom had been correct,Rutherford would have observed
A) alpha particles bouncing off the foil.
B) alpha particles going through the foil with little or no deflection.
C) alpha particles greatly deflected by the metal foil.
D) positive particles formed in the foil.
E) None of the above observations is consistent with the Thomson model of the atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass spectrum of an element with two naturally occurring isotopes is shown below.What is the best estimate of the element's atomic mass? 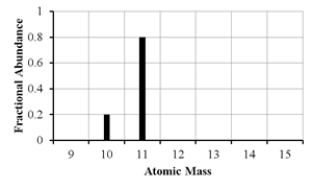
A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formulas of the hydroxide ion,the nitrate ion,and the phosphate ion are represented,respectively,as
A) OH-,NO2-,PO33-.
B) OH-,NO2-,PO43-.
C) H-,NO2-,P3-.
D) H-,NO3-,P3-.
E) OH-,NO3-,PO43-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a known ion?
A) S2+
B) Sc4+
C) Sn2+
D) P4-
E) Na-
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 149
Related Exams